Impact of IDO activation and alterations in the kynurenine pathway on hyperserotonemia, NAD+ production, and AhR activation in autism spectrum disorder
Published in Chemistry, Biomedical Research, and General & Internal Medicine
Autism spectrum disorder (ASD) is complex and heterogeneous. Symptomatic genetic mutations account for less than 10% of cases and more than 1000 genetic variants have been associated with ASD. More recently, the gut-brain axis has been involved in ASD1, although recent findings suggest that the gut microbiota is rather consequential of dietary behaviour than causal2. In marked contrast, metabolic parameters are currently less studied, even though the elevated level of blood serotonin (hyperserotonaemia) is the most frequent metabolic alteration observed, albeit only present in at most 40% of individuals with ASD 3. In ASD, hyperserotonaemia is characterised by an increase in platelet serotonin and results from a decrease in serotonin catabolism: reduced N-acetylation and methylation leading to a decrease of melatonin4 and mainly decreased sulphoconjugation by the monoamine-specific phenol sulphotransferase (PST-M)5.
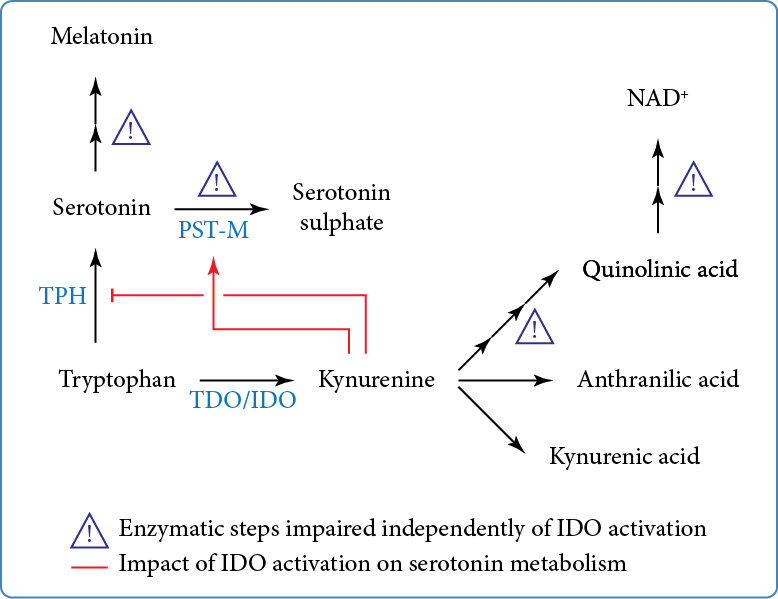
Serotonin is synthesised from the essential amino acid tryptophan (Figure). Tryptophan is also the precursor of the kynurenine pathway, which is initiated by the conversion of tryptophan to kynurenine. The kynurenine pathway is constitutively active in the liver where tryptophan is metabolised by tryptophan 2,3-dioxygenase. Upon inflammation, extrahepatic induction of the kynurenine pathway is initiated by indole 2,3 dioxygenase (IDO). Kynurenine is further catabolised into kynurenic, anthranilic, and quinolinic acids; the latter is the precursor for the de novo synthesis of NAD+.
In our recently published in Translational Psychiatry, we quantified by mass spectrometry the metabolites of the kynurenine pathway in the same cohort of individuals with ASD in which we reported extensive studies of the serotonin/melatonin pathway3-5.
We found that IDO is activated in almost 60% of individuals with ASD and is associated with cytokine levels consistent with low-grade inflammation. Given the antagonistic impact of IDO activation on serotonin synthesis, we also found that IDO activation resulted in ‘normal’ serotonin blood levels, through an increase in PST-M activity, possibly through its transcriptional regulation by the aryl hydrocarbon receptor. Importantly, when considering serotonaemia and IDO activation, almost 90% of individuals with ASD exhibited exclusively hyperserotonaemia and no IDO activation, or normoserotonaemia and IDO activation. Overall, our results indicate that the mechanisms underlying hyperserotonaemia are likely present in at least 90% of individuals with ASD and that one consequence of IDO activation is the restoration of serotonin catabolism by sulphoconjugation which eventually masks hyperserotonaemia.
In addition, we found various enzymatic anomalies in the kynurenine pathway, regardless of IDO activation (Figure). These anomalies add to the list of other enzymatic steps of tryptophan metabolism that are altered in ASD, namely the sulphoconjugation of serotonin and the deficit in melatonin production (Figure). Notably, the kynurenine pathway leads to the de novo synthesis of NAD+, which appeared lower in individuals with ASD. Importantly, strong negative correlations between NAD+ plasma levels with those of oxytocin and interleukin 1-β (only in individuals with ASD and IDO activation) strongly suggest that reduction in NAD+ synthesis has at least an impact on regulated secretion. Given the broad role of NAD+ in energy homeostasis, secretion, and epigenetic modifications, it can be anticipated that the decrease in NAD+ levels will have numerous metabolic consequences in ASD.
Taken together, these results extend biochemical anomalies of the tryptophan catabolism to the kynurenine pathway and posit TRP catabolism as a possible major component of ASD pathophysiology. Therefore, our data strongly suggest that ASD is mainly a metabolic disorder, way less heterogeneous than initially thought.
References
- Puricelli C, Rolla R, Gigliotti L, Boggio E, Beltrami E, Dianzani U et al. The Gut-Brain-Immune Axis in Autism Spectrum Disorders: A State-of-Art Report. Front Psychiatry 2021; 12: 755171.
- Yap CX, Henders AK, Alvares GA, Wood DLA, Krause L, Tyson GW et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021; 184(24): 5916-5931 e5917.
- Pagan C, Delorme R, Callebert J, Goubran-Botros H, Amsellem F, Drouot X et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl Psychiatry 2014; 4(11): e479.
- Pagan C, Goubran-Botros H, Delorme R, Benabou M, Lemiere N, Murray K et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci Rep 2017; 7(1): 2096.
- Pagan C, Benabou M, Leblond C, Cliquet F, Mathieu A, Lemiere N et al. Decreased phenol sulfotransferase activities associated with hyperserotonemia in autism spectrum disorders. Transl Psychiatry 2021; 11(1): 23.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in