Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient.
Published in Cancer

Background
Osimertinib is the cornerstone of advanced EGFR-mutant NSCLC management regardless of patients' previous treating status. Nevertheless, acquired drug resistance may prompt its discontinuation, with BRAF V600E driving disease progression in nearly 3% of the cases.1 Little is known with regards to the optimal treatment of these patients, with chemotherapy (with or without atezolizumab plus bevacizumab) remaining the treatment of choice in this unfavorable scenario.
Few reports have explored the activity of a combined EGFR/MAPK blockade in patients harboring BRAF alterations as acquired mechanisms of resistance (MR) to osimertinib, with a variety of dosage schedules and magnitude of benefit.2 Toxicities arising from these combinations are certainly hurdles to be overcome and a (justifiable) matter of concern, substantially differing among reports in the literature; consequently, lower starting doses and prescription of a reduced number of drugs (i.e. MEK inhibitors + osimertinib3 versus BRAFi + MEKi + osimertinib) have repeatedly been described in existing attempts to overcome BRAF-driven resistance while mitigating adverse events that could jeopardize patients' quality of life and treatment dose-intensity.
Equally important, temporal and spatial tumor heterogeneity demands a more comprehensive evaluation in order to identify these MR and accurately monitor treatment performance, as illustrated in the following figure. Blood-based ctDNA assessment (the so-called 'liquid biopsy'), by analyzing tumor DNA from multiple metastatic sites at once, is able to provide a deeper understanding of the molecular complexity of a given malignancy than single-site tissue biopsies. Sequential ctDNA assessments during the treatment may allow clinicians to better testify clonal evolution, identify targetable MR and anticipate response or radiological progression.
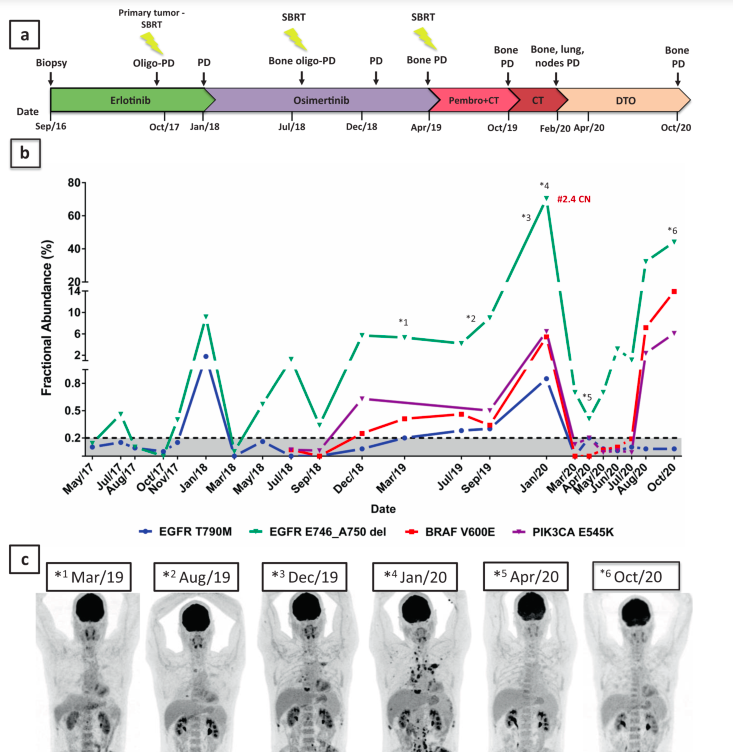
A successful attempt to overcome BRAF-driven resistance to osimertinib and monitor treatment performance through ctDNA assessment.
In the present article, we report an impressive response to combination dabrafenib, trametinib and osimertinib in a patient who developed a BRAF V600E, PIK3CA E545K, and EGFR amplification as MR to osimertinib. We employed droplet-digital PCR (ddPCR) as an additional surveillance method during the entire treatment, which demonstrated a valuable correlation with 18F-FDG-PET-CT imaging findings.
We described the case of a 50-year-old non-smoker male patient who had been diagnosed with a stage IV lung adenocarcinoma harboring EGFR del19 (E746_A750) in September 2016. Upon second-line osimertinib progression, in December 2018, a tissue-based NGS of a bone biopsy specimen disclosed BRAF V600E, EGFR amplification, and PIK3CA E545K. In January 2020, following approximately one year of chemotherapy-based regimens, he presented at our clinic complaining of severe bone pain and fatigue.
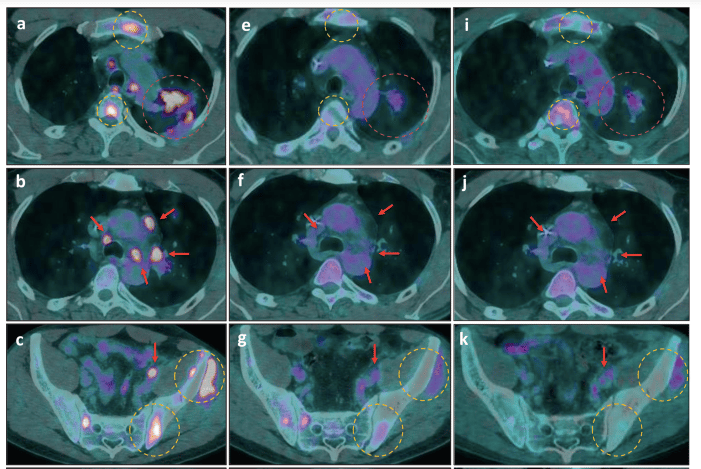
After revisiting his previous NGS report (and considering the patient´s good performance status), we prescribed dabrafenib 75mg bid, trametinib 1mg OD and osimertinib 80mg OD. He experienced remarkable pain relief within 2 weeks of treatment, with only mild treatment-related toxicities. His radiological response in the first follow-up PET-CT was impressive, with nearly 70% tumor burden reduction as assessed by PERCIST criteria (Figure 2 - panels e, f, and g). He remained progression-free until October when mild progression occurred. Throughout his entire treatment, longitudinal ddPCR ctDNA assessment demonstrated its interesting and useful concordance with PET-CT scan, which underscores this methodology´s potential to anticipate imaging findings.





Photos include: Dr. Maurício Ribeiro, Dr. Artur Katz, Dr. Anamaria Aranha Camargo, Dr. Franciele Knebel, Dr. João Victor Alessi, Dr. Karina Sacardo and Dr. Andrea Shimada, all from Hospital Sírio-Libanês - Oncology Center, São Paulo, Brazil.
For further information, the original article is available at www.nature.com/articles/s41698-021-00149-4.
References:
1. Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019 Nov;137:113-122. doi: 10.1016/j.lungcan.2019.09.017. Epub 2019 Sep 23. PMID: 31568888; PMCID: PMC7478849.
2. Meng P, Koopman B, Kok K, Ter Elst A, Schuuring E, van Kempen LC, Timens W, Hiltermann TJN, Groen HJM, van den Berg A, van der Wekken AJ. Combined osimertinib, dabrafenib and trametinib treatment for advanced non-small-cell lung cancer patients with an osimertinib-induced BRAF V600E mutation. Lung Cancer. 2020 Aug;146:358-361. doi: 10.1016/j.lungcan.2020.05.036. Epub 2020 Jun 4. PMID: 32534795.
3. Dagogo-Jack I, Piotrowska Z, Cobb R, Banwait M, Lennerz JK, Hata AN, Digumarthy SR, Sequist LV. Response to the Combination of Osimertinib and Trametinib in a Patient With EGFR-Mutant NSCLC Harboring an Acquired BRAF Fusion. J Thorac Oncol. 2019 Oct;14(10):e226-e228. doi: 10.1016/j.jtho.2019.05.046. PMID: 31558234.
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
AI Approaches in Drug Design
Publishing Model: Open Access
Deadline: Mar 31, 2026
Genomic Instability
Publishing Model: Open Access
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in