In situ observation of a stepwise [2 + 2] photocycloaddition process using fluorescence spectroscopy
Published in Chemistry
Solid-state [2+2] photocycloaddition reactions of olefinic compounds have been demonstrated to represent a powerful access to organic cyclic molecules with specific configurations that are difficult to be obtained using solution reaction methods1. We have investigated the applications of solid-state [2+2] photocycloaddition reactions of coordination polymers (CPs) for the stereoselective synthesis of cyclobutane derivatives in our laboratory2. However, in order to understand the reaction process and better control these reactions, it has become increasingly important to research the mechanism behind them, which has not been well developed.
The appropriate analytical techniques3 for solid-state [2+2] photocycloaddition reactions include infrared spectroscopy (IR), Raman spectroscopy, nuclear magnetic resonance spectrometer (NMR), powder X-ray diffraction (PXRD), thermal methods, single crystal X-ray diffraction (SCXRD) and so on. The Raman spectroscopy and IR absorption peak position and intensity can be used to identify the structural composition and quantitatively analyze the content of chemical groups. However, owing to the overlapping of C=C bonds and the weak C-C vibrational peak, the application of Raman spectroscopy and IR in the [2+2] photocycloaddition reaction is limited. In-situ NMR has become one of the important measures of modern analysis and research, but in situ solid-state NMR is rarely reported and its resolution is low. Thermal analysis mainly depends on the differences in the weight loss of different photoaddition products. However, it took almost 1 hour to collect one data point. In addition, excessive heating often triggers the reverse (thermal) reaction of dissociation. The in situ XRD technique includes single crystal X-ray diffraction and powder X-ray diffraction, which is based on Bragg diffraction. To date, the most precise method to characterize the structural transformations of CPs is single-crystal X-ray diffraction. However, in most cases, single crystals of CPs do not withstand the internal stress resulting from external stimuli and lose the single-crystal character required for SCXRD investigation. And when the proportion of cyclobutane products generated is small under short-term illumination, the low spatial resolution and time resolution of XRD technique makes it impossible to detect the local sites or components4. Our experimental investigations were also greatly impacted by the shortcomings of these analytical techniques. Therefore, it is challenging to find an in-situ characterization technique with highly sensitive and short response time for detecting the dynamic evolution of reaction intermediates.
Incidentally, when CP1 was irradiated under ultraviolet light, we observed a clear fluorescence enhancement, and this anomaly caught our attention. Then we investigated the photoreaction process by 19F NMR spectra. And the results showed very exciting correlation between the photoreaction process and the fluorescence intensity change, which hinted the photocycloaddition induced this change. Therefore, we designed in situ fluorescence spectroscopy to monitor the fluorescence changes of the two-step photocycloaddition reaction. By comparing the fluorescence intensity changes with the results of 19F NMR, the purpose of monitoring the solid-state [2+2] photocycloaddition reactions progress was successfully accomplished by in situ fluorescence spectroscopy. Because the π-conjugated system is modified during the [2+2] photocycloaddition process, all three CPs display fluorescence with different intensities and quantum yields (QYs). Thus, CP1-1 with a higher intramolecular through-space conjugation (TSC) displays the largest intensity fluorescence and QY compared to the other two compounds. The amount of cyclobutane is very small when CP1 irradiated with UV light for a very short time, the fluorescence emission intensity still changes significantly. As shown in the Fig. 1, since the fluorescence properties of the CP1, CP1-1 and CP1-2β are different, the fluorescence intensity changes accordingly with the change of photoproducts, and the fluorescence intensity is enhanced as the conversion of CP1-1 becomes larger.
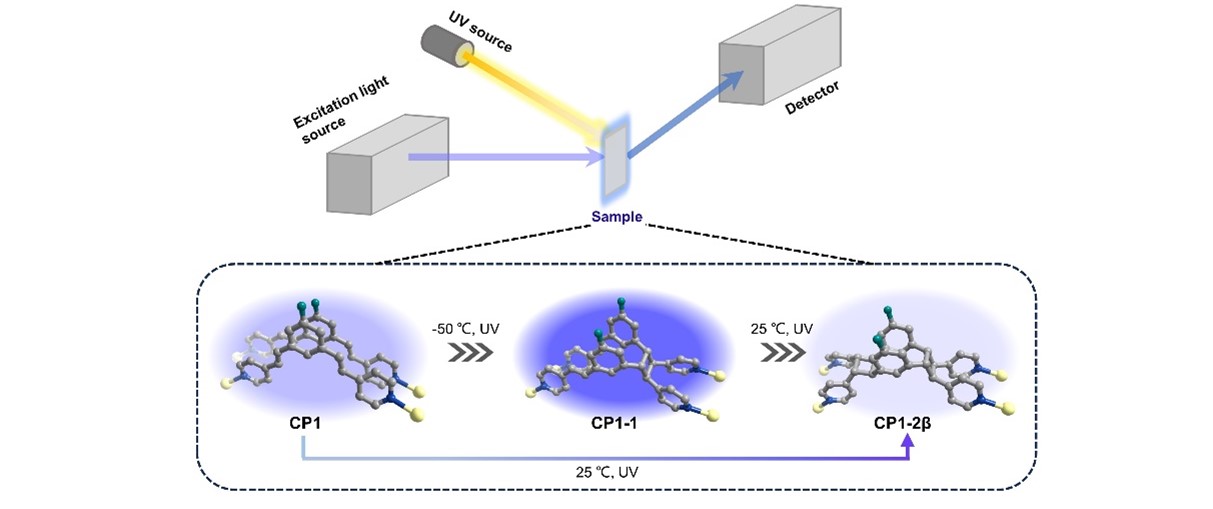
Fig. 1│Schematic diagram of in situ fluorescence spectroscopy and structures of the two-step [2+2] photocycloaddition transformation of CP1 to CP1-2β.
In addition, laser scanning confocal microscopy (LSCM) images of CP1 irradiated under UV light were collected at room temperature and analyzed to directly visualize the fluorescence changes within a single crystal during the [2+2] photocycloaddition reaction process. the LSCM results clearly indicate that the [2+2] photocycloaddition reaction started from the top of the crystal (UV-exposed side) and gradually reached the bottom layers. This is not achieved by the mentioned analytical techniques above.
In this study, we present, for the first time, the in situ observation of the photocycloaddition process using fluorescence spectroscopy involving a controllable two-step [2+2] photocycloaddition in a CP platform. The high sensitivity of fluorescence spectroscopy allows changes to be monitored even when the yield of cyclobutane product is very low and UV light irradiation maintained for a short time. We believe this work not only provides a powerful strategy for the visualization of [2+2] photocycloaddition process but also may open new possibilities for the kinetic study of a variety of fast reactions.
References
- Macgillivray, L. R. et al. Supramolecular control of reactivity in the solid state: from templates to ladderanes to metal organic frameworks. Chem. Res. 41, 280-291 (2008).
- Wang, M. F. et al. Coordination-driven stereospecific control strategy for pure cycloisomers in solid-state diene photocycloaddition. Am. Chem. Soc. 142, 700-704 (2020).
- Lyu, J. et al. Phase transitions in metal-organic frameworks directly monitored through in situ variable temperature liquid-cell transmission electron microscopy and in situ X-ray diffraction. Am. Chem. Soc. 142, 4609-4615 (2020).
- Li, X., Wang, S., Li, L., Sun, Y. & Xie, Y. Progress and perspective for in situ studies of CO2 J. Am. Chem. Soc. 142, 9567-9581 (2020).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in