Installation of Synergistic Binding Sites onto Porous Organic Polymers for Efficient Removal of Perfluorooctanoic Acid
Published in Chemistry

Per-and poly-fluoroalkyl substances (PFAS), as environmental pollutants, often show an adverse and severe impacts on environmental and human health due to their intrinsic high toxicity, extraordinary prevalence and persistence, and instant bioaccumulation. According to the U.S. Environmental Protection Agency (EPA), the PFAS concentration for combined perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in drinking water should not exceed the healthy advisory level of 70 ppt. Conventional PFAS adsorbents often have some limitations including low capacity, long equilibrium time, weak binding affinity, poor water/chemical stabilities, and low natural organic matter (NOM) selectivity, which limit their practical applications in the treatment of contaminated water. Therefore, it is an urgent task to develop advanced technologies for PFAS removal from contaminated water.
To overcome these drawbacks, it is essential to develop ideal types of PFAS-based adsorbents that possess the following features: (i) high hydrophilicity of the adsorbent particles and densely accessible functionalized capturing sites, benefiting for the well water wettability to facilitate the mass transfer of PFOA toward adsorbents in aqueous solutions and thereby achieving high PFAS uptake capacity in water; (ii) strong interactions with PFAS that can accelerate the adsorption rate; (iii) suitable pore size to avoid the co-adsorption of natural organic matter (e.g. humic acid, HA) in contaminated water during the adsorption process; (iv) exceptional water/chemical stability to facilitate regeneration/recyclability. Considering these factors, porous organic polymers (POPs), with porous aromatic framework (PAF) as the representative, were employed as a platform to construct PFAS adsorbent due to their physical and chemical properties. A synergistic binding sites protocol was illustrated to construct PFAS adsorbent by simultaneously introducing electrostatic and hydrophobic sites onto POPs as adsorption sites (Fig. 1).
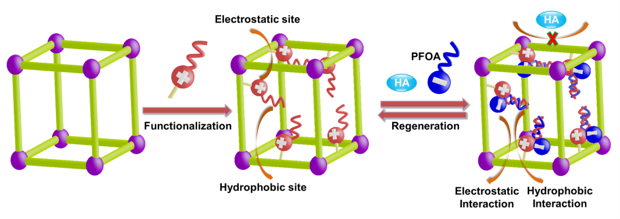
Fig. 1 ‘Synergistic binding sites’ strategy for PFOA removal. Illustration of ‘Synergistic binding sites’ strategy to construct highly efficient sorbents for PFOA removal. (HA represents humic acid, a model compound of organic co-contaminants or natural organic matters).
As a proof of concept, reported herein is a superior PFAS adsorbent by appending quaternary ammonium groups with long hydrophobic chains [e.g. N,N-dimethylpropylamine (NDMP), N,N-dimethyl-butylamine (NDMB), N,N-dimethylhexylamine (NDMH)] onto the pore wall of PAF-1 to obtain PAF-1-NDMP, PAF-1-NDMB, and PAF-1-NDMH, respectively (Fig. 2).
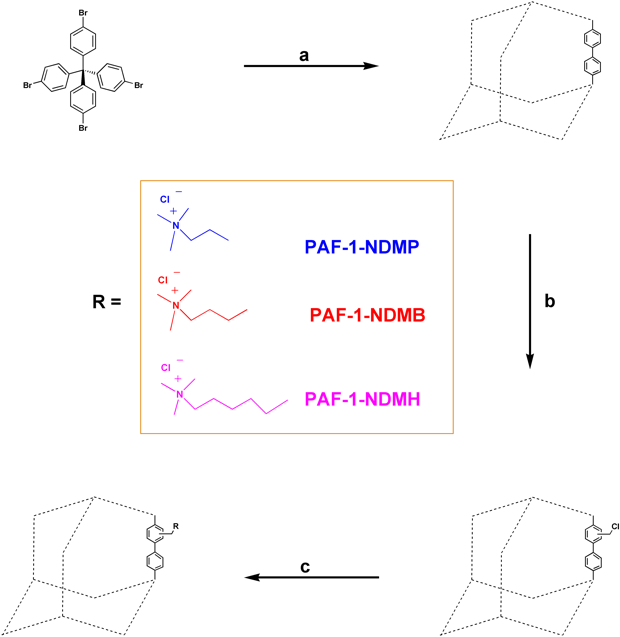
Fig. 2 General procedure for the synthesis of PFOA adsorbents including PAF-1-NDMP, PAF-1-NDMB, PAF-1-NDMH.Reaction conditions: a Bis(1,5-cyclooctadiene)nickel(0), 1,5-cyclooctadiene, 2,2’-bipyridine, N,N-dimethylformamide, and tetrahydrofuran, room temperature. b Paraformaldehyde, AcOH, H3PO4, HCl, 90 °C. and c N,N-dimethylpropylamine (NDMP), N,N-dimethyl-butylamine (NDMB), or N,N-dimethylhexylamine (NDMH) in EtOH, 90 °C.
As a result, PAF-1-NDMB as an optimized adsorbent exhibits extremely fast kinetics with the highest k2 value of 24000 g mg−1h−1 among all reported sorbent materials and a record high saturation PFOA (a model pollutant among PFAS) uptake capacity of over 2000 mg g-1 (Fig. 3a, 3b and 3c). The results of breakthrough experiment, in the present of HA (20 ppm), proved that PAF-1-NDMB exhibits highly efficient removal efficiency under the conditions of both low concentration (initial concentration: 500 ppb) and high concentration (initial concentration: 400 ppm) of PFOA, which is 14.6 and 19 times higher than that of benchmark material DFB-CDP under the same conditions (Fig. 4a and 4b). Besides, PAF-1-NDMB can remove 99.99% PFOA in practical wastewater (collected from Xiaoqing River, which is the most heavily PFOA polluted water area in China) within 10 s and reduce PFOA concentrations from initial concentration of 166.27 ppt to < 70 ppb (EPA advisory level) within 10 s (Fig. 4c). This proof-of-concept study is important because it can be readily extended to sorts of POPs, we believe that a large amount of POP-based PFOA sorbents would be emerged in the near future and thus realize the practical application for decontaminating PFAS from polluted water.
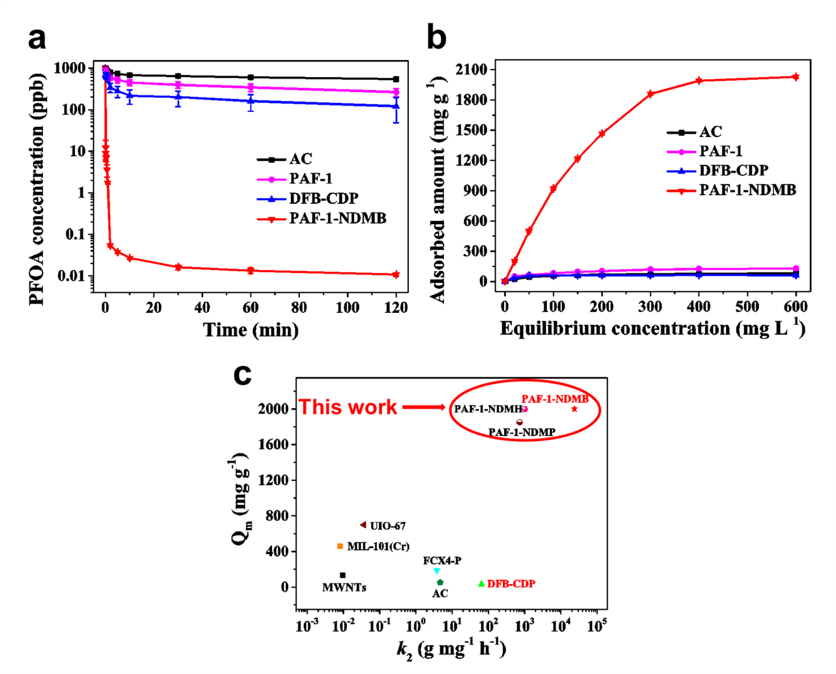
Fig. 3 Kinetics, adsorption isotherm investigation of PAF-1-NDMB and comparison of PFOA saturation uptake amount and k2 value for PAF-1-NDMB with other benchmark porous materials. a PFOA sorption kinetics of AC, PAF-1, DFB-CDP, and PAF-1-NDMB. b Plotting of equilibrium PFOA adsorption capacity as a function of equilibrium PFOA concentration. c The comparison of PFOA saturation uptake amount and k2 value for PAF-1-NDMB with other threshold porous materials, including DFB-CDP; MIL-101(Cr); MWNTs; FCX4-P; UIO-67; AC. Red color represents that the materials show PFOA selectivity under the existence of NOM. All the error bars in this figure represent the standard deviation (SD, n = 3 independent experiments), data are presented as mean values ± SD.
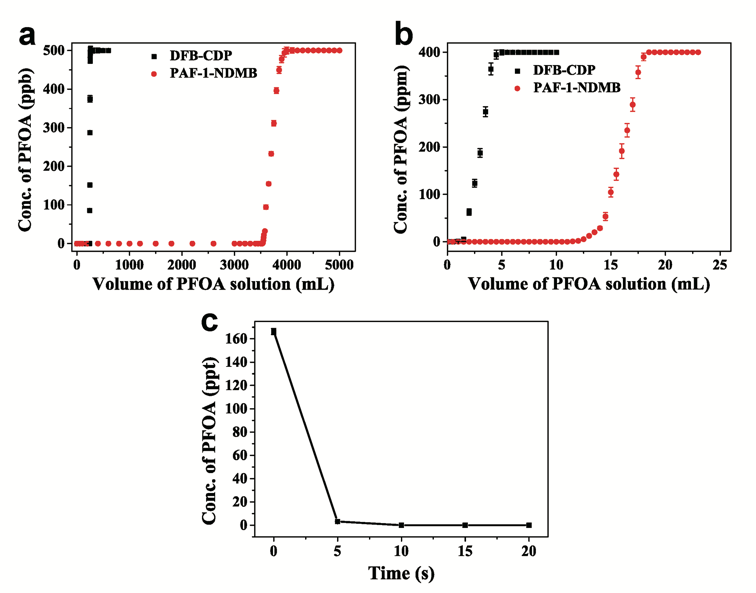
Fig. 4 Breakthrough experiments and practical environmental contaminated water adsorption experiments. Breakthrough experiments of a PAF-1-NDMB and DFB-CDP in aqueous PFOA/HA solutions (PFOA 500 ppb, HA 20 ppm). bPAF-1-NDMB and DFB-CDP in aqueous PFOA/HA solutions (PFOA 400 ppm, HA 20 ppm). c Adsorption performance of PAF-1-NDMB in removing practical contaminated water (initial concentration with 166.27 ppt). All the error bars in this figure represent the standard deviation (n = 3 independent experiments), data are presented as mean values ± SD.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in