Tuning excited state electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance
Published in Chemistry

The development of highly active and low-cost COF photocatalysts is now an international research focus. To obtain high-performance COF photocatalysts, researchers are now exploring ways of increasing the visible-light absorption range, optimizing band structures, and decreasing the recombination of photogenerated electrons and holes. Common strategies for achieving these performance-boosting properties include (i) incorporating a photosensitizer into the framework for improving the light-harvesting capability; (ii) functionalization of the linkers and tuning of the components to optimize the band gap energy and valence/conduction band potentials; (iii) construction of donor-acceptor moieties to improve charge transfer kinetics and charge carrier separation efficiencies; (iv) doping non-metal elements (N, P, S, etc.), single metal sites, clusters, or noble metals as a co-catalyst to modulate the photoelectronic properties, thus improving the overall photocatalytic activity. These approaches demonstrate that controlling electron energy levels and electron transport in COFs is vital to improving photocatalytic performance.
COF-based adsorbent-photocatalyst systems are now being pursued for uranium extraction. However, the developed systems have relatively low activity for U(VI) reduction and/or need sacrificial reagents for separating photo-generated charges. Further, their complicated structures result in indeterminacy of catalytic sites, hindering efforts to understand reaction mechanisms that would enable rational COF photocatalyst design. A further unmet challenge in this domain is the limited sunlight absorption and charge carrier utilization efficiency, both of which depend on COF structure and electron transport pathways at a molecular level (Figs. 1a and 1b). Decreasing the energy losses during electron transfer from the excited state to the acceptor (e.g., adsorbed UO22+) while reducing fluorescence emissions are essential for improving photocatalytic performance (Figs. 1a and 1b).
Herein, we synthesized a series of isoreticular COFs, named as COF-1, COF-2, COF-3 and COF-4, with different excited state electron distributions, charge transport properties and local pore characteristics (Fig. 1c), which we subsequently evaluated for the photocatalytic reduction of U(VI) to U(IV) solid (such as UO2) from seawater and contaminated groundwater.
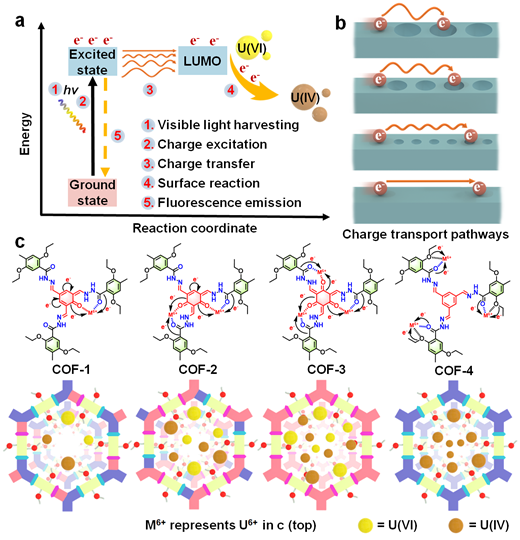
Fig. 1 | Overview of the electron excitation processes and charge carrier utilization in COFs. (a) Schematic illustration of charge carrier separation and utilization by COF photocatalysts, highlighting the energy levels involved in the charge transfer processes. (b) Illustration of charge transfer from a COF donor to a U(VI) acceptor (highlighting the different utilization efficiency), which can be optimized by tuning the excited state electronic distribution. (c) Structures of COF-1 to COF-4 with different components in the pores to modulate the excited state electronic structure and charge transport properties, thus tuning the photocatalytic activities for uranium extraction.
The optoelectronic properties of the obtained COFs were evaluated. As shown in Figs. 2a and 2b, COF-3 and COF-4 have the smallest semicircle diameter, the strongest photocurrent responses than COF-1 and COF-2. All the obtained COFs possessed good light harvesting ability, electron conductivity, charge separation and transport properties, particularly COF-4. The difference in these properties between the COFs could be attributed to the local pore characteristics decorated with varying components attached to the photoactive units. This suggested that the electron carrier utilization efficiency in the COF photocatalysts could be systematically tuned by controlling and regulating the local pore characteristics, thus enhancing their photocatalytic performance.
To explore the practical potential of our approach, we next performed detailed photocatalytic uranium extraction studies on the aforementioned COFs in spiked seawater, groundwater, and natural seawater. No sacrificial electron donor reagents were used in the reaction system. COF-4 quickly removed uranium from spiked seawater, with an uptake capacity of 182 mg/g U achieved over 24 h, larger than the other three ones (Fig. 2c). COF-4 exhibited rapid uranium removal performance in natural seawater with an uptake capacity as high as 20.6 mg/g after 72 h, which was significantly higher than that of all COFs reported to date, with a super high extraction efficiency of 6.84 mg/g/day (Fig. 2e). Our results show that significant improvements in photocatalytic performance can be achieved by tuning the local pore characteristics through linker modification in COFs. This motivated a deep investigation of the relationship between COF structure and photocatalytic activity.
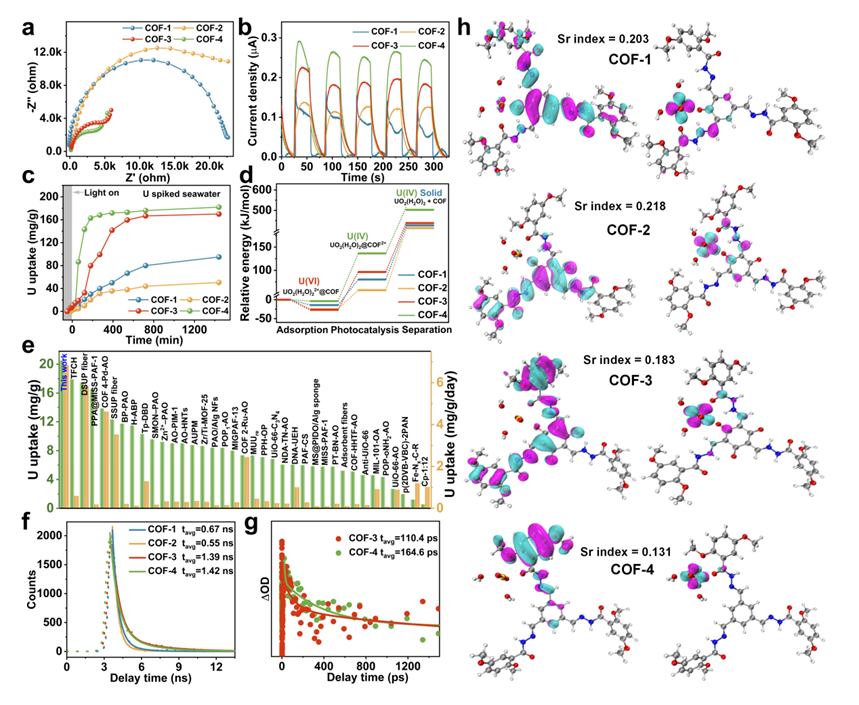
Fig. 2 | Optoelectronic properties, U(VI) removal efficiency and DFT calculation results. (a) Electrochemical impedance spectra (EIS) of COF-1, COF-2, COF-3, and COF-4. (b) Transient current density of COF-1, COF-2, COF-3, and COF-4. (c) Uranium extraction from spiked seawater with initial uranium concentrations of ~20 ppm, using COF-1, COF-2, COF-3, and COF-4 as photocatalysts. (d) The relative free energy diagrams of uranium adsorption and reduction on COF photocatalysts. (e) Comparison of uranium extraction performance of COF-4 and other reported materials in natural seawater. (f) PL lifetime of COF photocatalysts. (g) Femtosecond time-resolved transient absorption decay kinetics of COF photocatalysts. (h) The S1 excited state electronic structures of COF photocatalysts (highlighting hole-electron distribution).
To obtain deeper insights into the photocatalytic mechanism, we carried out photoluminescence (PL) lifetime, transient absorption spectra (TAS) measurements, and density functional theory (DFT) calculations on the COFs to verify the electron excitation states, electronic donation sites, and transport pathways. COF-4 have longer PL life time and larger average TAS lifetime than COF-3 (Figs. 2f and 2g), revealing COF-4 showed slower electron-hole combination kinetics and better interfacial charge separation and migration in their extended conjugated skeletons, which was beneficial for improving its photocatalytic performance. According to DFT calculation results, COF-4 has the highest visible light utilization ability (Fig. 2d), and the most efficiently charge carrier separation (Fig. 2h). From the results of Hirshfeld charges and bond length changes, one can find that the structure of the COFs controlled the mechanism of electronic transfer during photocatalytic U(VI) reduction.
In summary, we report a new design strategy for constructing highly conjugated hydrazide-based COF photocatalysts with unique optoelectronic characteristics and outstanding photocatalytic activities. By optimizing the excited state electron distribution, electronic donation sites, electron transportation and local pore characteristics in the COFs, photocatalytic U(VI) extraction from seawater and wastewater could be maximized, and the factors influencing the photocatalytic properties of the COFs could be understood. One of our COFs, COF-4, possessed an extremely high uranium uptake capacity of 6.84 mg/g/day, state-of-the-art performance for any COF-based adsorbent or adsorbent-photocatalyst in natural seawater. The new mechanistic insights this work provides about charge separation and transport in COFs during photocatalysts support the rational design of new COFs for uranium extraction and other photocatalytic applications.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in