Integrating metal catalysis into photoelectrochemical systems to create new reaction paradigms
Published in Chemistry
Needs and Challenges
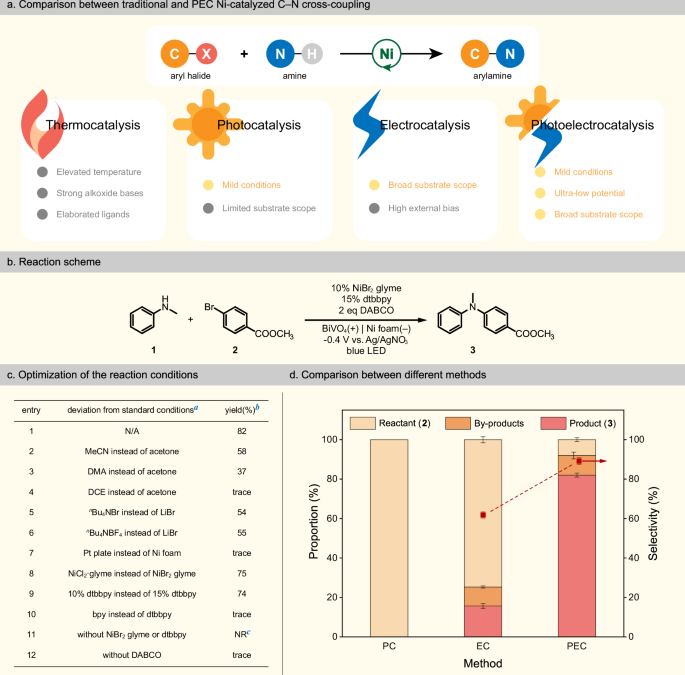
As we compared photoelectrocatalysis (PEC) with other methods like photocatalysis (PC) and electrocatalysis (EC), its potential is prominent. PEC occurs at the electrode interface, eliminating the need for catalyst separation and reducing costs. The introduction of light helps us overcome energy barriers, making organic reactions more energy-efficient. However, drawbacks exist: the limited reaction types, low conversion efficiency, and poor stability of current photoelectrocatalytic organic synthesis (PECOS) systems. It became clear to us that there is an urgent need to diversify the types of reactions, for which integrating efficient metal catalysis into the photoelectrochemical reaction system to create a new paradigm is one of the most promising candidate methodologies.
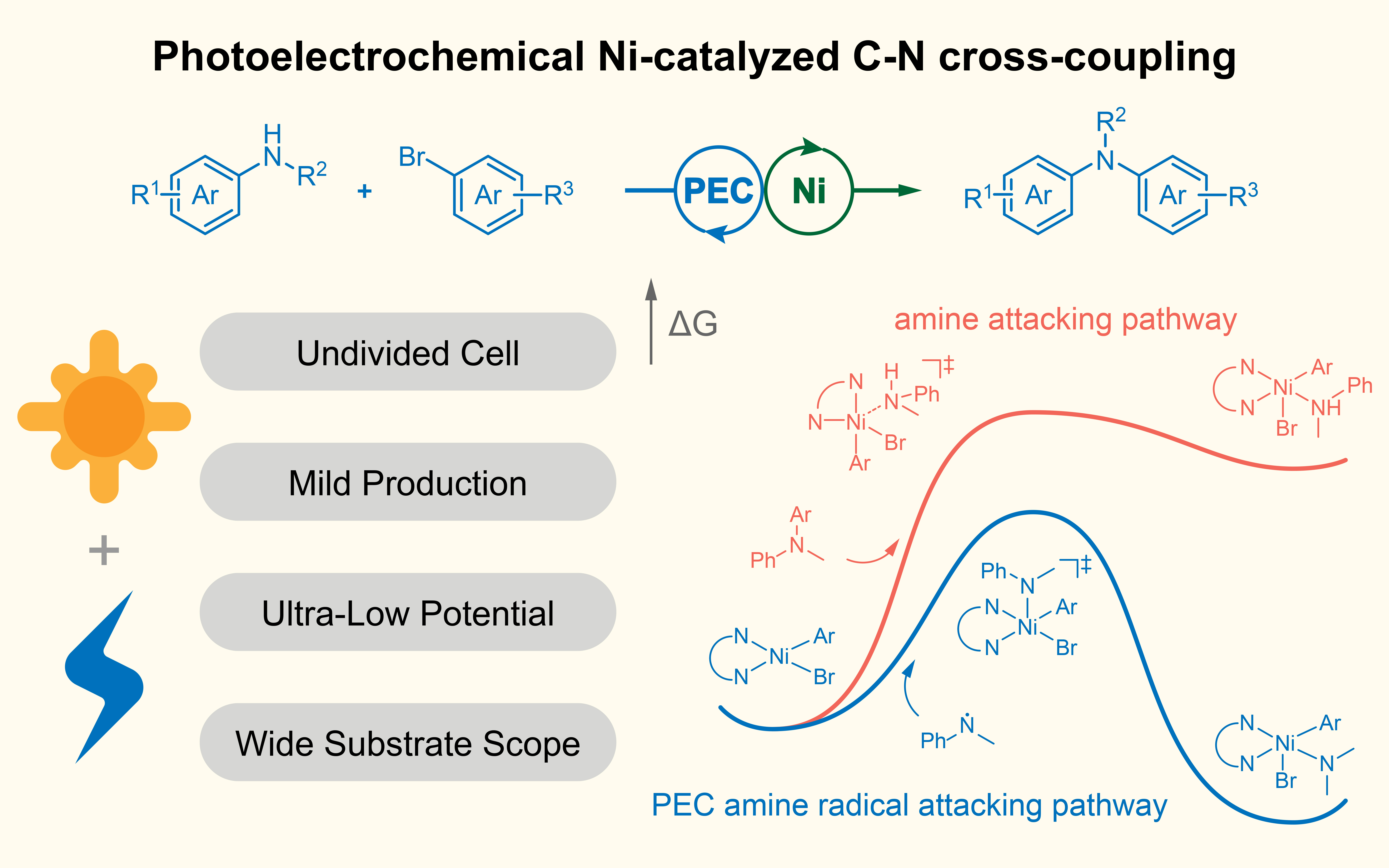
Fig. 1 Photoelectrochemical Ni-catalyzed cross-coupling of aryl bromides with amine.
Bridging PECOS with metal catalysis
With this goal in mind, we introduce nickel catalysis into PECOS and realize the cross-coupling reaction between N-methylaniline and methyl 4-bromobenzoate. Besides the nickel catalyst, the system includes a three-electrode setup where BiVO4 acts as the photoanode, nickel foam serves as the counter electrode, and Ag/AgNO3 is used as reference electrode. A hydrogen atom transfer (HAT) mediator DABCO is also added to the system to facilitate the C–H activation. Our control experiments confirmed the crucial roles played by the nickel catalyst, ligand, and DABCO in the catalytic process. When we assessed the contributions of light and electricity, we found that under light irradiation alone, no target product was detected. Under electrolysis alone, the yield was a mere 16%, and a high bias was needed. These findings highlighted the essence of both light and electricity in this catalytic process. Notably, under PEC conditions, we were able to significantly reduce the voltage to –0.4 V vs. Ag/AgNO3 while achieving an 82% product yield.
Nature of the Bridge
To further understand EC and PEC properties of our reaction system, we sorted to linear sweep voltammetry (LSV) and cyclic voltammetry (CV). The results were enlightening. The onset potential of the BiVO4 photoanode was significantly lower compared to the glassy carbon anode. When we employ the glassy carbon anode for the same reaction, only a small amount of the target product at 1.0 V vs. Ag/AgNO3 was observed, underscoring the superiority of PEC system. Introducing DABCO contributed to a reduction in potential, acting as a mediator that facilitated charge transfer between the photoanode and the substrate. We also noted that the oxidation potential of DABCO was substantially lower than that of N-methylaniline, indicating that DABCO was more easily oxidized at the anode. This observation was consistent with our LSV results, which showed a pronounced increase in the oxidation peak intensity of DABCO in the presence of N-methylaniline, suggesting an interaction between oxidized DABCO and N-methylaniline. Additionally, our CV tests revealed changes in the oxidation state of the Ni catalyst. We observed a reduction peak at E = –1.73 V vs. Ag/AgNO3, attributed to the Ni(II/I) signal. After adding aryl bromide, the reduction peak intensity significantly increased, indicating the oxidative addition of aryl bromide to the Ni(I) species.
With these insights, we proposed a mechanism for the photoelectrochemical nickel-catalyzed C-N coupling reaction. Under visible light irradiation, electrons in the valence band (VB) of the photoanode are excited to the conduction band (CB) and then migrate to the cathode, initiating the nickel catalytic cycle. The photogenerated holes oxidize DABCO into a cationic radical through single-electron transfer, which then interacts with the amine substrate through hydrogen atom transfer, generating amine radical. The amine radical subsequently participates in the nickel catalytic cycle to form the target product. Our DFT calculations revealed that, compared to the traditional electrocatalytic pathway where the amine directly attacks, the amine radical pathway has a lower energy barrier, highlighting the advantage of photoelectrochemical nickel catalysis.
The Broad Road and beyond
As we expanded the scope of substrates, we observed fair to good yields with aryl halides featuring different functional group substitutions. However, we also noted significant variations in the catalytic performance for various amine derivatives, likely due to the influence of electronic effects and steric hindrance on the stability of nitrogen radicals. Encouragingly, pharmaceutical molecules like canagliflozin, bedaquiline, fenofibrate, and indomethacin were also compatible with this reaction system, demonstrating the potential applications of PECOS.
Our journey into the realm of PECOS has not only deepened our understanding but opened new avenues for innovation in organic chemistry. We are excited about the future possibilities and the potential impact of our work on sustainable chemical synthesis.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in