Isoxazole-based molecules restore NK cell immune surveillance in hepatocarcinogenesis by targeting TM4SF5 and SLAMF7 linkage
Published in Biomedical Research
-
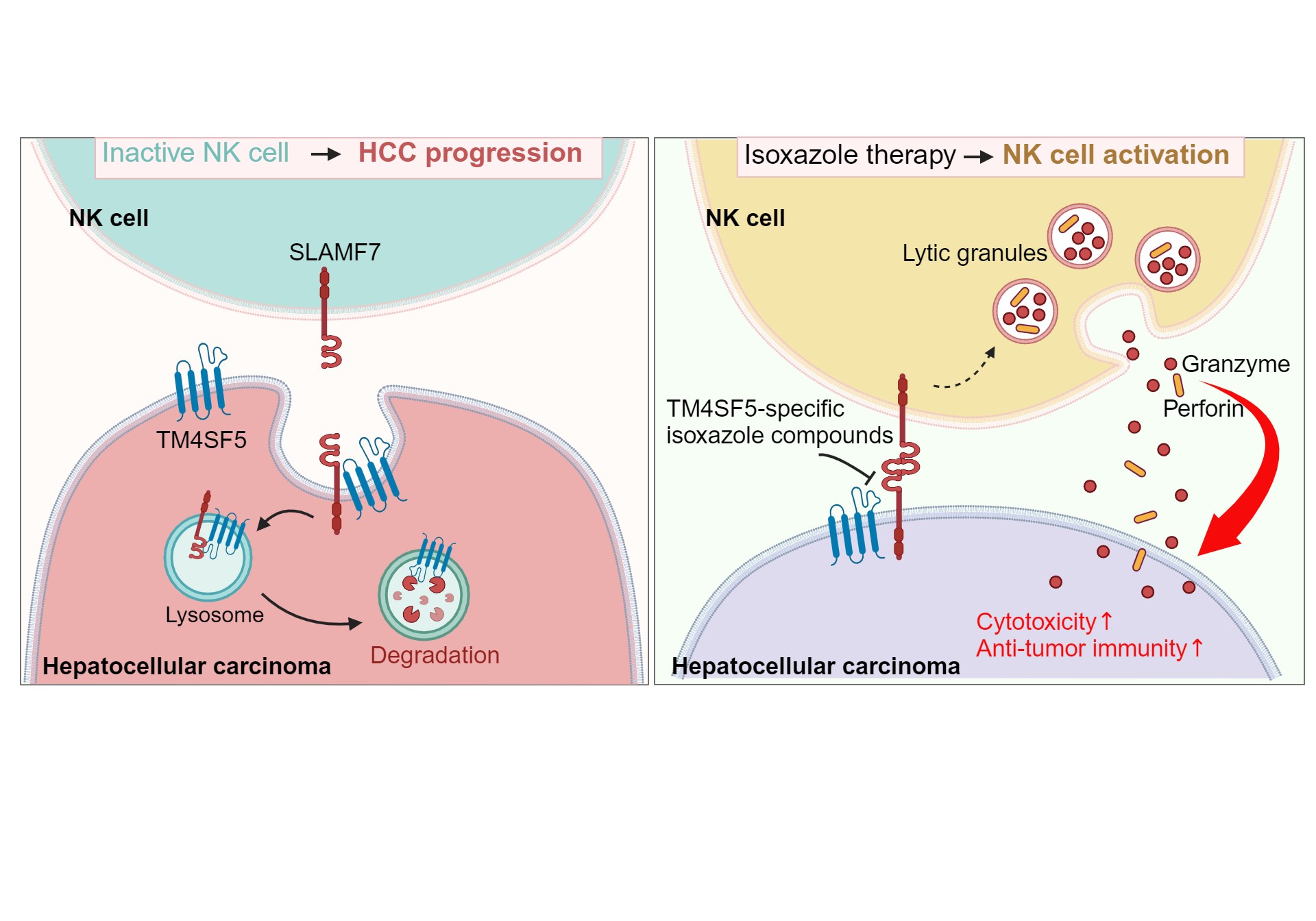 Immunotherapy is a type of cancer treatment that helps your immune system to fight cancer cells. Supporting T cell immunity is the major strategy of immunotherapy, but recently diverse researches have been performed to boost other types of immune cells. Here, we have focused on the new immunotherapy by enhancing cytotoxicity of natural killer cells (NK cells) for liver cancer cell treatment that has been limited with T cell-based immunotherapy alone. We previously reported that transmembrane 4 L six family member 5 (TM4SF5) promotes HCC development and avoids immune surveillance by NK cells, although molecular mechanisms remained to be explored. Recently, we also identified TM4SF5-specific isoxazoles based small molecules (TSIs) by comprehensive structure activity relationship analysis. We have aimed to figure out molecular mechanism of restoration of NK cell immune surveillance by TSIs and verify the anti-tumor effects of the drug in liver cancer models.
Immunotherapy is a type of cancer treatment that helps your immune system to fight cancer cells. Supporting T cell immunity is the major strategy of immunotherapy, but recently diverse researches have been performed to boost other types of immune cells. Here, we have focused on the new immunotherapy by enhancing cytotoxicity of natural killer cells (NK cells) for liver cancer cell treatment that has been limited with T cell-based immunotherapy alone. We previously reported that transmembrane 4 L six family member 5 (TM4SF5) promotes HCC development and avoids immune surveillance by NK cells, although molecular mechanisms remained to be explored. Recently, we also identified TM4SF5-specific isoxazoles based small molecules (TSIs) by comprehensive structure activity relationship analysis. We have aimed to figure out molecular mechanism of restoration of NK cell immune surveillance by TSIs and verify the anti-tumor effects of the drug in liver cancer models.
TM4SF5-mediated hepatocellular carcinogenesis
In our previous researches focused on the TM4SF5-mediated tumorigenesis, we found that TM4SF5 is overexpressed in cancerous hepatocytes of various mice models and human patients, and also avoids immune surveillance. Building on this, we aimed to demonstrate the molecular mechanism driving tumorigenesis by uncovering TM4SF5-expressing hepatocyte and immune cell communication via immune ligand-receptor interactions. To address the clinical significance of close relevance of TM4SF5 and specific NK cell immune ligands, we performed survival analysis using human liver cancer patient samples. Looking at data from the TCGA-LIHC (The Cancer Genome Atlas – liver hepatocellular carcinoma), we found that patient with strong TM4SF5 expression was linked to lower SLAMF7 expression, resulting in poor survival rates. However, other NK cell ligands did not have significant relevance with TM4SF5 for survival probability. These clinical data analyses suggest that TM4SF5 might promote HCC development by downregulating SLAMF7 expression which could negatively affect NK cell activity, in addition to observations from animal and patient HCC tissue analyses.
Development of new chemical inhibitor
The chalcone-based TM4SF5 inhibitor, TSAHC (4’-[p-toluenesulfonylamido]-4-hydroxychalcone), shows anti-tumor effects for treating TM4SF5-related HCC in mice. However, it shows also lower bioavailability, thereby leading to possible limitations in the in vivo efficacy and off-target effects. As a result, we newly developed brand-new version of compounds and identified TM4SF5-specific isoxazoles based small molecules (TSIs). To examine the therapeutic effects of TSIs, we established several in vivo mouse liver cancer models – carcinogen (DEN)-induced HCC, xenografts, and HCC PDX model, using male or female mice with immune-deficient or competent background. The efficacy of TSIs was verified in various HCC models and showed efficient effects to block the tumor growth via NK cell surveillance.
Mechanistic insights
Previously, we reported that TM4SF5 localizes at both plasma membrane and lysosomal membrane depending on extracellular environment. Cellular functions and the interacting proteins become differed between two localizations. The translocation of TM4SF5 is induced by cellular energy status – in glucose or arginine fast state, TM4SF5 localizes at plasma membrane. Repletion of glucose or arginine induce translocation of TM4SF5 from plasma membrane to lysosomal membrane. However, the relevance of TM4SF5 localization and immune regulatory function and activity has not been explored until this study. To understand the mechanism of restoring NK cell immune surveillance in HCC by targeting TM4SF5 and SLAMF7 linkage, we performed in vitro western blot, immunofluorescence assay, immunoprecipitation, and flow cytometry, in addition to diverse in vivo animal studies.
The results of western blot reveal that the protein level of SLAMF7 is lower in TM4SF5 expressing cells compared to TM4SF5-negative cells. After mRNA translation, the SLAMF7 protein could be translocated to lysosome together with TM4SF5 via their association depending on N-glycosylation on either side, resulting in SLAMF7 loss. Immunofluorescence assay showed that SLAMF7 favors lysosomal membrane in TM4SF5-positive cells, followed by the lysosomal degradation of SLAMF7. Either impaired N-glycosylation in TM4SF5 or SLAMF7 or pharmacological inhibition of lysosomal degradation activity or TM4SF5 function prevented TM4SF5-mediated SLAMF7 loss. A molecular docking model showed TSIs to associate with TM4SF5 N-glycosylation reside, and isotope-labelled TSI was shown to directly bind TM4SF5 in in vitro biding assay. TSI-mediated inhibition of the interaction of TM4SF5 with SLAMF7 blocked the lysosomal degradation of SLAMF7. Thus, the TSIs treatment to in vitro cells and in vivo animal models eventually led to recovery of NK cell activity, resulting in less tumor features or formations.
Conclusions
Our finding demonstrates a novel mechanism of TM4SF5-mediated immune checkpoint regulation by which the expression of SLAMF7, one of the stimulatory NK ligands, is regulated via N-glycosylation dependent binding to TM4SF5, cellular trafficking, and lysosomal degradation. The regulation of NK ligand expression contributes to the interaction of cancer and immune cells and eventually anti-tumor immunity of surveillance. Our research uncovers the molecular mechanism of hepatocyte and NK cell communication, and also suggest a new therapeutic target for killing the liver cancer cells and blocking the progression.
References
1. Tetraspanin TM4SF5 mediates loss of contact inhibition through epithelial-mesenchymal transition in human hepatocarcinoma. J Clin Invest. 2008 Apr;118(4):1354-66.
2. TM4SF5-mediated liver malignancy involves NK cell exhaustion-like phenotypes. Cell Mol Life Sci. 2021 Dec 18;79(1):49.
3. Differential ligand binding/trafficking for distinct CTLA-4 fates: is it an expandable mechanism?. Cell Mol Immunol. 2023 Jan;20(1):1-2.
4. Glucose-mediated mitochondrial reprogramming by cholesterol export at TM4SF5-enriched mitochondria-lysosome contact sites. Cancer Commun (Lond). 2024 Jan;44(1):47-75.
Follow the Topic
-
Signal Transduction and Targeted Therapy

This is an international, peer-reviewed, open-access journal publishing articles related to signal transduction in physiological and pathological processes, alongside signal transduction-targeted therapeutics in the form of biological agents and small molecular drugs used to treat human diseases.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in