Less Stain, More Gain: Using AI to Reduce Extra Tests in Prostate Histopathology
Published in Protocols & Methods, Computational Sciences, and General & Internal Medicine

Digitizing Pathology: Paving the Way for Smarter Prostate Cancer Diagnosis
Histopathology is currently undergoing a digital revolution. Many laboratories are transitioning from traditional microscopes to digitized biopsy slides, which can be viewed in high-resolution on computer screens. This shift enables new possibilities, including the integration of artificial intelligence (AI) into diagnostic workflows.
Every day, pathologists around the world diagnose prostate cancer by assessing digital hematoxylin and eosin (H&E)-stained slides. Routine H&E stains reveal both the overall tissue architecture and the details of individual cells, allowing trained eyes to distinguish between benign and malignant changes.
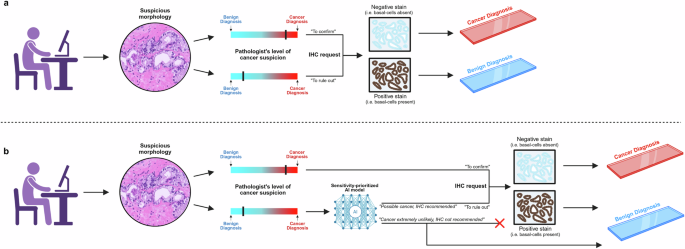
But sometimes, the answer is not entirely clear-cut. Certain glands appear atypical, sitting somewhere between normal and cancerous. In these situations, pathologists often choose caution over risk – even when they strongly suspect that a biopsy is benign, many will still order immunohistochemical (IHC) stains to remove any lingering doubt.
IHC can highlight the presence or absence of specific proteins, helping to confirm or rule out cancer. However, it comes at the cost of increased laboratory workload, use of expensive reagents, and delays the final diagnosis. In many cases, the extra staining turns out to have been unnecessary in retrospect: the H&E slide alone would have been sufficient, but caution drives overuse.
This raised a question for us:
Could artificial intelligence serve as an alternative to IHC in confirming benign tissue morphology in cases where pathologist suspicion of cancer is low?
The Challenge: Diagnosing the Difficult Cases
AI has already shown impressive performance in detecting prostate cancer on H&E slides, with several published models achieving near-perfect sensitivity and specificity in validation studies. However, most of these studies have looked at the overall AI performance, without focusing specifically on the truly challenging or borderline biopsies that trigger IHC investigations in everyday practice. What happens when we look only at these cases where the morphology is ambiguous - the kinds of cases that even experienced pathologists find challenging?
This question was at the heart of our study. Here, we focused exclusively on difficult diagnostic scenarios: prostate core needle biopsies where the original pathologists had needed IHC to reach a final diagnosis. If AI performs well here, it could have great potential in reducing unnecessary IHC use and easing pressure on busy labs.
Building and Testing the AI Model
In our earlier work, we developed and validated an AI model to detect and grade prostate cancer on H&E-stained slides, drawing on roughly 100,000 prostate biopsies from patients across the globe. To understand how the AI performs on truly difficult cases, we went back through our validation datasets and pulled out all samples where the original pathologists had ordered IHC—a clear sign that the morphology on H&E alone hadn’t been enough for a confident diagnosis.
The model’s task was simple enough:
Predict whether a slide is benign using only the H&E image, and decide if IHC can be safely avoided.
If we want pathologists to trust the AI’s benign predictions and skip IHC staining, the model has to be virtually flawless when it comes to sensitivity. In other words, it must minimize the risk of calling a cancerous slide benign, since that would compromise patient safety.
To make sure of this, we set classification thresholds that prioritize sensitivity. Essentially, the model is designed to be cautious when uncertain and only predict “benign” when it is extremely confident that no cancer is present.
Here’s how it works: for each slide, the AI predicts a cancer probability between 0% and 100%. By adjusting the classification threshold, we control the cut-off at which a slide is predicted as “malignant”, or in this context, “not definitively benign”. A default threshold of 50% wouldn’t work here — some cancers would inevitably be misclassified as benign. Instead, we use much lower thresholds, for example, 1%. This means that even a tiny hint of cancer in the AI’s prediction will flag the case as “not definitively benign,” ensuring it gets IHC to clear up any uncertainty.
Crucially, when the AI predicts a case as benign, it is doing so with extremely high certainty. The key question then becomes: can it do this often enough in tricky cases to reduce IHC use by a meaningful amount?
Moment of Truth
We were greatly anticipating the results. Could the AI really handle these challenging biopsies as reliably as we hoped? As we began analyzing the AI predictions, the answer quickly became clear: the model performed exceptionally well. It distinguished malignant from benign slides with astonishing accuracy, far exceeding what we had dared to expect in such difficult cases.
To our relief, the threshold adjustments had worked exactly as intended – applying a classification threshold of 1%, there was not a single cancer misclassified as benign among all slides analyzed by the AI. Furthermore, despite the heavy sensitivity-prioritization, the model was still able to classify most benign slides correctly. Put another way – in many cases found difficult by the pathologists, the AI found no difficulty at all.
The results suggested that a significant portion of IHC stains could have been avoided – up to 80.6% for benign slides – highlighting the potential savings of using such a tool in the diagnostic process.
For us, the most reassuring finding was that the AI never made a false negative prediction. In a field where patient safety is paramount, minimizing false negatives was a non-negotiable requirement, and seeing the model meet it gave us real confidence in its potential. These results didn’t just confirm our expectations; they hinted at a future where AI could meaningfully streamline workflows, reduce unnecessary testing, and allow pathologists to focus their attention where it’s needed most.
The Road to Clinical Implementation
Although the results are promising, there’s still a journey ahead before this approach can become part of everyday clinical practice. Real-world deployment will require rigorous prospective testing, careful integration into existing workflows, and navigation of regulatory pathways to ensure patient safety and trust.
This study represents a meaningful step forward, showing that AI can support pathologists in precisely the kinds of cases where uncertainty often drives overuse of IHC. If we can bring this technology into practice, the future of prostate histopathology may truly be one of less stain — and more gain.
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in