Light, Circadian Clock and Mood--Linked Together by Human Genetic Variants
Published in Neuroscience

Circadian rhythms are the ~24h rhythm manifest by our behavior and physiological processess including sleep/wakefulness, hormone release, heart rate, blood pressure, etc. These rhythms are driven by endogenous circadian clocks, which at the molecular level, consist of a number of genes (termed clock genes) that form transcriptional/translational feedback loops 1. Even our mood displays fluctuation on daily bases, although how this is regulated is still unknown 2. Moreover, it has been known for decades that mood disorders such as major depressive disorder, bipolar disorder and seasonal affective disorder (SAD) are accompanied by circadian disruptions, while the underlying mechanisms are largely unclear 3.
We became interested in the relationship between circadian clock and mood when we encountered a family with genetic variants in the circadian clock gene PERIOD3 (PER3) that result in alterations of two amino acid residues (PER3-P415A/H417R). Individuals carrying these variants are affected by SAD, which is characterized by depressive episodes in winter months with spontaneous remission in the spring/summer 4. SAD is induced by lack of daylight in the winter when day length (or photoperiod) is short, but the pathogenic mechanism remains elusive. Therefore, these naturally occurring PER3 variants provide a key to find out more regarding this enigmatic disorder.
We adopted the bacterial artificial chromosome technology to generate transgenic mice carrying either wild-type (WT) or mutant human PER3 gene. This renders PER3 to be expressed following its endogenous pattern, which is more or less ubiquitous expression throughout the body. We then subjected these animals to either a short photoperiod condition with 4h light/20h dark (4L20D) each day or a regular photoperiod with 12h light/12h dark (12L12D). The mutant transgenic animals display significantly increased despair like behaviors compared to WT transgenic, and this difference is more prominent under 4L20D, mimicking the symptoms of SAD patients. Light therapy delivered in the early morning is the most commonly used treatment for SAD, and this manipulation can also rescue the despair-like phenotype of PER3-P415A/H417R mice. In addition, clinical study has reported a potential brain serotonergic deficit in SAD patients 5. Therefore, we administered selective serotonin reuptake inhibitor (SSRI, which boosts serotonergic signaling) to these animals and observed a rescue of the despair-like phenotype as well. Taken together, PER3-P415A/H417R mice display similarities with SAD patients both at the behavioral and pharmacological level, strongly suggesting that these variants are indeed the cause of SAD in the human carriers.
Upon successful establishment of the SAD mouse model, we next explored the mechanism by which PER3-P415A/H417R leads to winter depression-like behaviors. The effectiveness of SSRI in relieving the despair-like phenotype implicates potential serotonergic deficiency in PER3-P415A/H417R animals. Therefore, we examined the expression of Tryptophan hydroxylase 2 (Tph2), a rate-limiting enzyme of the serotonin synthesis pathway, in the dorsal raphe nucleus (DR) which is a major source of serotoninergic neurons in the brain. We found that Tph2 transcription is down-regulated in PER3-P415A/H417R mice selectively under 4L20D but not 12L12D. We subsequently conducted genetic rescue to validate that the despair-like phenotype is indeed caused by lack of Tph2.
For quite a while we were troubled by how PER3-P415A/H417R inhibits Tph2 transcription. Since the most (and perhaps only) well-characterized function of PER3 is to modulate transcription, we tested whether PER3 is involved in transcriptional regulation of Tph2 but were not able to obtain any positive result. In a stress assay, we noticed that PER3-P415A/H417R animals exhibits elevated corticosterone level in the circulation. A couple studies have reported that corticosterone and its receptor, glucocorticoid receptor, can inhibit Tph2 expression in the DR 6. Therefore, we suspected that the excessive corticosterone in PER3-P415A/H417R mice may be the cause of decreased Tph2 transcription and despair-like phenotype. This was later confirmed by pharmacological treatments to increase or suppress glucocorticoid signaling and adrenolectomy.
We have previously assumed that PER3-P415A/H417R must be acting in the brain to induce winter depression-like phenotype, but these corticosterone-related findings point to a completely different direction—the primary site of action is likely to be the adrenal gland (AG) which synthesizes corticosterone. Adrenocorticotropic hormone (ACTH), synthesized and released by the pituitary gland, binds to receptors on the adrenal cortex and elicits a series of downstream signaling events. During this process, adrenal cells uptake cholesterols and transfer them into the mitochondria where corticosterone is synthesized. We found that the AGs of PER3-P415A/H417R animals are more sensitive to ACTH, i.e. they synthesize more corticosterone upon ACTH stimulation. Moreover, when we expressed WT or mutant human PER3 only in the adrenal cortex, we were able to recapitulate the winter depression-like phenotype, accompanied by systemic elevation of corticosterone. In depth molecular analysis revealed that PER3 protein binds to steroid acute regulatory protein (StAR), which transports cholesterol from the cytoplasm into the mitochondria and serves as a rate-limiting factor during steroidogenesis. The mutant PER3 protein stabilizes StAR, resulting in an increase of its level, potentially contributing to the enhanced corticosterone synthesis.
The question that remains is how short photoperiod triggers despair-like phenotype in PER3-P415A/H417R animals. We found that blood corticosterone level is down regulated in PER3-WT mice as photoperiod shortens, but this down-regulation fails to occur in PER3-P415A/H417R mice. In fact, the hypothalamic-pituitary-adrenal axis is toned down under short photoperiod, as ACTH level is reduced under 4L20D vs. 12L12D. However, as mentioned above, PER3-P415A/H417R renders the AG more sensitive to ACTH stimulation. Therefore, although ACTH is down-regulated in PER3-P415A/H417R animals under 4L20D, corticosterone is still maintained at a level comparable to that of 12L12D and thus resulting in increased corticosterone selectively under 4L20D.
Although our results thus far can explain how PER3-P415A/H417R leads to winter depression-like phenotype under short photoperiod, one puzzling issue that emerges is why the elevated corticosterone level under longer photoperiod (elevated relative to that under short photoperiod) does not result in despair-like phenotype. We noticed that in both WT and mutant transgenic animals, DR TPH2 expression is higher under 12L12D compared to 4L20D, despite the fact that corticosterone level is higher under 12L12D. This indicates that light can in some way activate TPH2 expression to counteract the inhibition imposed by corticosterone. Indeed, seasonal modulation of serotonin with an increase in long summer days compared to short winter days have been reported in human and rodents 7-10. However, the underlying mechanism is yet unknown, which is what we are currently exploring and hopefully will come to an answer in the near future.
Taken together, our work here starts from human PER3 variants associated with SAD and identify a permissive role for these variants in promoting corticosterone synthesis. Seasonal changes of photoperiod, on the other hand, serves as an instructive signal to up regulate TPH2 level under longer photoperiod and down regulate it under short photoperiod. When challenged with excessive corticosterone under short winter-like photoperiod when TPH2 expression is already low, this will perhaps push the system past a certain threshold and ultimately resulting in depression-like behaviors.

Related references:
1 Li, S. & Zhang, L. Circadian Control of Global Transcription. Biomed Res Int 2015, 187809, doi:10.1155/2015/187809 (2015).
2 Emens, J. S. et al. Circadian rhythm in negative affect: Implications for mood disorders. Psychiatry Res 293, 113337, doi:10.1016/j.psychres.2020.113337 (2020).
3 Dollish, H. K., Tsyglakova, M. & McClung, C. A. Circadian rhythms and mood disorders: Time to see the light. Neuron 112, 25-40, doi:10.1016/j.neuron.2023.09.023 (2024).
4 Zhang, L. et al. A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait. Proc Natl Acad Sci U S A, doi:10.1073/pnas.1600039113 (2016).
5 Levitan, R. D. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues Clin Neurosci 9, 315-324 (2007).
6 Vincent, M. Y., Donner, N. C., Smith, D. G., Lowry, C. A. & Jacobson, L. Dorsal raphe nucleus glucocorticoid receptors inhibit tph2 gene expression in male C57BL/6J mice. Neurosci Lett 665, 48-53, doi:10.1016/j.neulet.2017.11.041 (2018).
7 Carlsson, A., Svennerholm, L. & Winblad, B. Seasonal and Circadian Monoamine Variations in Human Brains Examined Post Mortem. Acta Psychiatr Scand 61, 75-85, doi:10.1111/acps.1980.61.s280.75 (1980).
8 Brewerton, T. D., Berrettini, W. H., Nurnberger, J. I., Jr. & Linnoila, M. Analysis of seasonal fluctuations of CSF monoamine metabolites and neuropeptides in normal controls: findings with 5HIAA and HVA. Psychiatry Res 23, 257-265, doi:10.1016/0165-1781(88)90016-9 (1988).
9 Lambert, G. W., Reid, C., Kaye, D. M., Jennings, G. L. & Esler, M. D. Effect of sunlight and season on serotonin turnover in the brain. Lancet 360, 1840-1842, doi:10.1016/s0140-6736(02)11737-5 (2002).
10 Goda, R. et al. Serotonin levels in the dorsal raphe nuclei of both chipmunks and mice are enhanced by long photoperiod, but brain dopamine level response to photoperiod is species-specific. Neurosci Lett 593, 95-100, doi:10.1016/j.neulet.2015.03.035 (2015).
Follow the Topic
-
Nature Metabolism

This journal publishes work from across all fields of metabolism research that significantly advances our understanding of metabolic and homeostatic processes in a cellular or broader physiological context, from fundamental cell biology to basic biomedical and translational research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement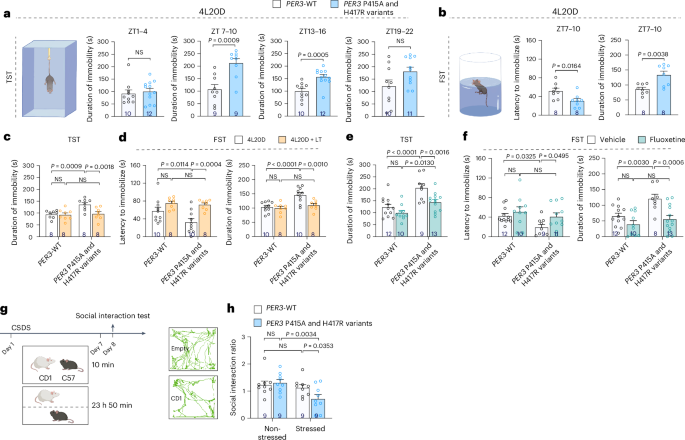

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in