α-lipoic acid modulates prostate cancer cell growth and bone cell differentiation
Published in Cancer
Concern in Mind (Prostate cancer and Bone metastasis)
Prostate cancer is a prevalent and primary contributor to cancer-related fatalities, and the incidence of metastases directly influences prognostic and clinical outcomes. Patients diagnosed with advanced prostate cancer frequently experience bone metastases, which can result in skeletal fractures and pain. Despite notable progress in the treatment of original tumors, metastatic instances of prostate cancer continue to be associated with a high mortality rate. In prostate cancer, the most identified locations of bone metastases are the spine, pelvis, and ribs [1, 2]. Bone metastasis is a multi-step process, from the establishment of cancer cells in bone to their reactivation and remodeling of bone tissue, ultimately leading to structural and functional alterations [3].
α-Lipoic Acid as a Nutraceutical
α-lipoic acid [ALA] is a naturally occurring potent bioactive compound with promising anti-neoplastic effects across various cancer types. Synthesized enzymatically in the mitochondria, ALA primarily serves as a cofactor for numerous mitochondrial metabolic enzymes, underscoring its physiological importance [4, 5]. While de novo synthesis of ALA fulfills physiological needs, it is also abundant in meats, dark green, and leafy vegetables, making them valuable nutritional sources. Furthermore, in addition to its anti-cancer properties, ALA supplementation enhances insulin sensitivity in rats by alleviating oxidative stress [6-10].
Unlocking ALA's Potential in Prostate Cancer and Bone Metastases
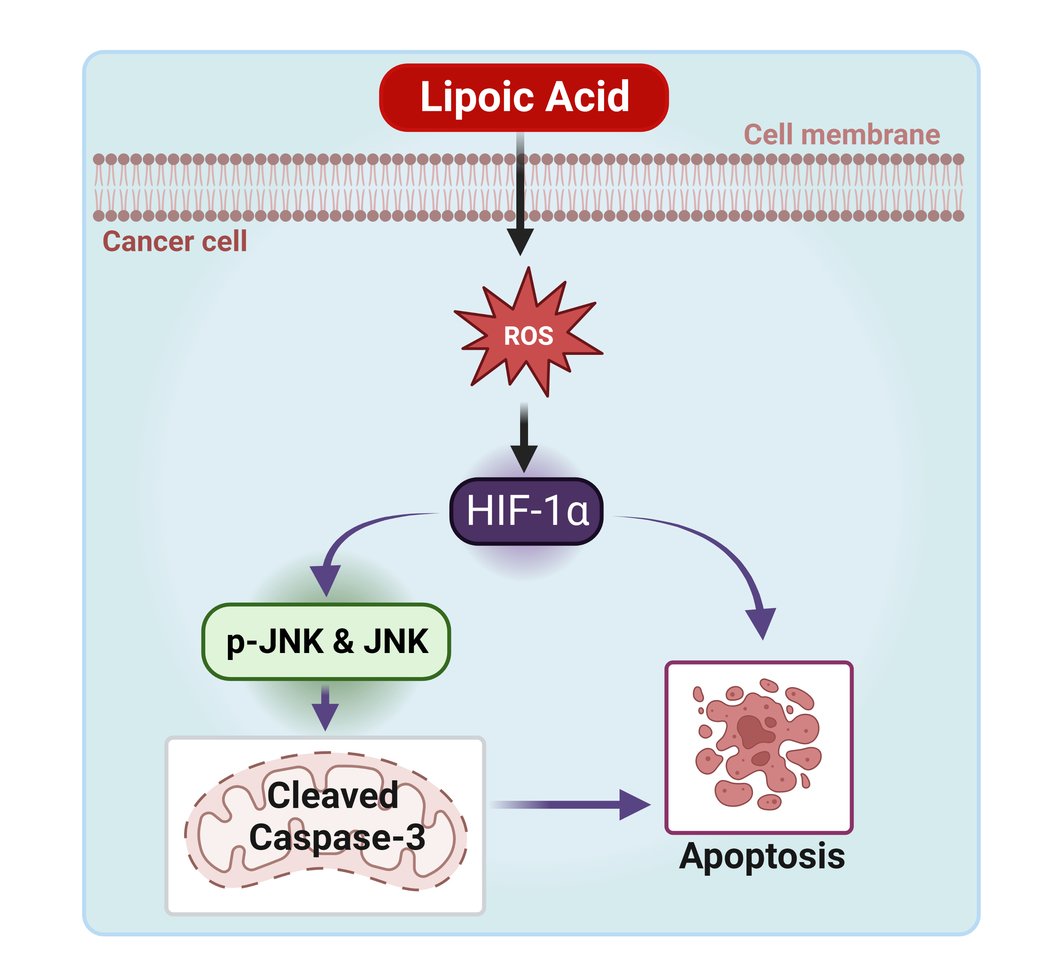
A team of eight researchers led by Dr. Jawed Siddiqui from the Department of Biochemistry and Molecular Biology at the University of Nebraska Medical Center elucidates the mechanism underlying ALA's anti-neoplastic action in prostate cancer to bone metastases. For the first time, we demonstrated that ALA supplementation inhibits prostate cancer cell motility and invasion by triggering death via ROS production. Moreover, ALA supplementation attenuates the impact of cancer secretome on bone cells, mitigating Prostate cancer-mediated bone cell growth and resorption.
Exciting Outcomes
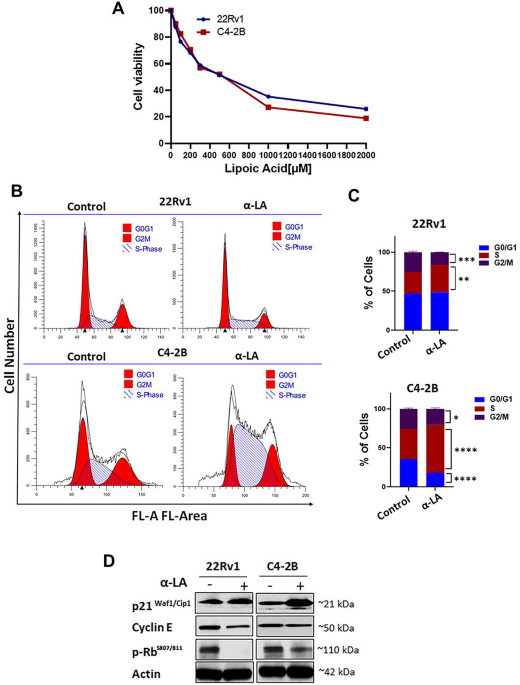
ALA treatment resulted in significantly lower cell viability of prostate cancer cells in a dose-dependent manner, accompanied by a considerably higher percentage of apoptosis compared to untreated cells, as evidenced by elevated expression of apoptotic markers. The cell death caused by ALA treatment is due to the production of ROS, which triggers the HIF1α/JNK/Caspase-3 pathway in prostate cancer cells. Notably, functional studies with ALA indicate a reversal of the EMT process, associated with reduced migratory and invasive capabilities of cancer cells. Furthermore, ALA treatment downregulates the expression of p-ERK, suggesting inhibition of survival signaling pathways. Additionally, ALA treatment modulates bone cell differentiation within prostate cancer microenvironment. Overall, ALA supplementation promotes bone health by limiting the interaction of cancer cells and the bone microenvironment. These findings indicate a novel mode of action for ALA and serve as a starting point for developing nutraceutical-based therapies for prostate cancer patients with bone metastases.
References
- Kakhki, V.R., et al., Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev Cent East Eur, 2013. 16(2): p. 66-9.
- Zhang, X., Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun (Lond), 2019. 39(1): p. 76.
- Croucher, P.I., M.M. McDonald, and T.J. Martin, Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer, 2016. 16(6): p. 373-86.
- Herbert, A.A. and J.R. Guest, Lipoic acid content of Escherichia coli and other microorganisms. Arch Microbiol, 1975. 106(3): p. 259-66.
- Shay, K.P., et al., Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta, 2009. 1790(10): p. 1149-60.
- Kataoka, H., N. Hirabayashi, and M. Makita, Analysis of lipoic acid by gas chromatography with flame photometric detection. Methods Enzymol, 1997. 279: p. 166-76.
- Farhat, D., et al., Lipoic acid decreases breast cancer cell proliferation by inhibiting IGF-1R via furin downregulation. Br J Cancer, 2020. 122(6): p. 885-894.
- Peng, P., et al., Alpha-lipoic acid inhibits lung cancer growth via mTOR-mediated autophagy inhibition. FEBS Open Bio, 2020. 10(4): p. 607-618.
- Thirunavukkarasu, V., A.T. Anitha Nandhini, and C.V. Anuradha, Cardiac lipids and antioxidant status in high fructose rats and the effect of alpha-lipoic acid. Nutr Metab Cardiovasc Dis, 2004. 14(6): p. 351-7.
- Chakravarti, B., et al., Lipoic acid blocks autophagic flux and impairs cellular bioenergetics in breast cancer and reduces stemness. Biochim Biophys Acta Mol Basis Dis, 2022. 1868(10): p. 166455.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in