Locked Aminoboranes for Unlocking Persistent Phosphorescence and Chiral Luminescence

From Synthesis to Function: Navigating Challenges, Crystallization, and Application
Our group has been exploring BN/CC isosteres for more than a decade. The B-N moiety is isoelectronic and structurally similar to C=C moieties; however, the electronegativity difference between B and N blesses B-N embedded systems with distinctive optical characteristics, such as through-bond CT. Their optical properties can be further modulated by controlling the molecular conformations or donor/acceptor strength and the number of D/A moieties. Moreover, the B-N moiety blesses them with excited states enduring different symmetries (π-π* and n–π*), thus facilitating effective SOC and enhancing the spin flipping (ISC and reverse ISC) process. Furthermore, the inherent difference in their orbital and electronic configurations aids them in creating an excited state with the right symmetry (π-π* and n-π*), which offers high spin-orbit coupling (SOC) and enhances ISC and rISC, leading to an efficient delayed luminescence. By exploiting the intriguing optical and electronic signatures of aminoboranes, we have demonstrated aggregation-induced emission enhancement, triboluminescence, mechanoluminescence, deep blue delayed fluorescence, and p-RTP (persistent room-temperature phosphorescence), etc.
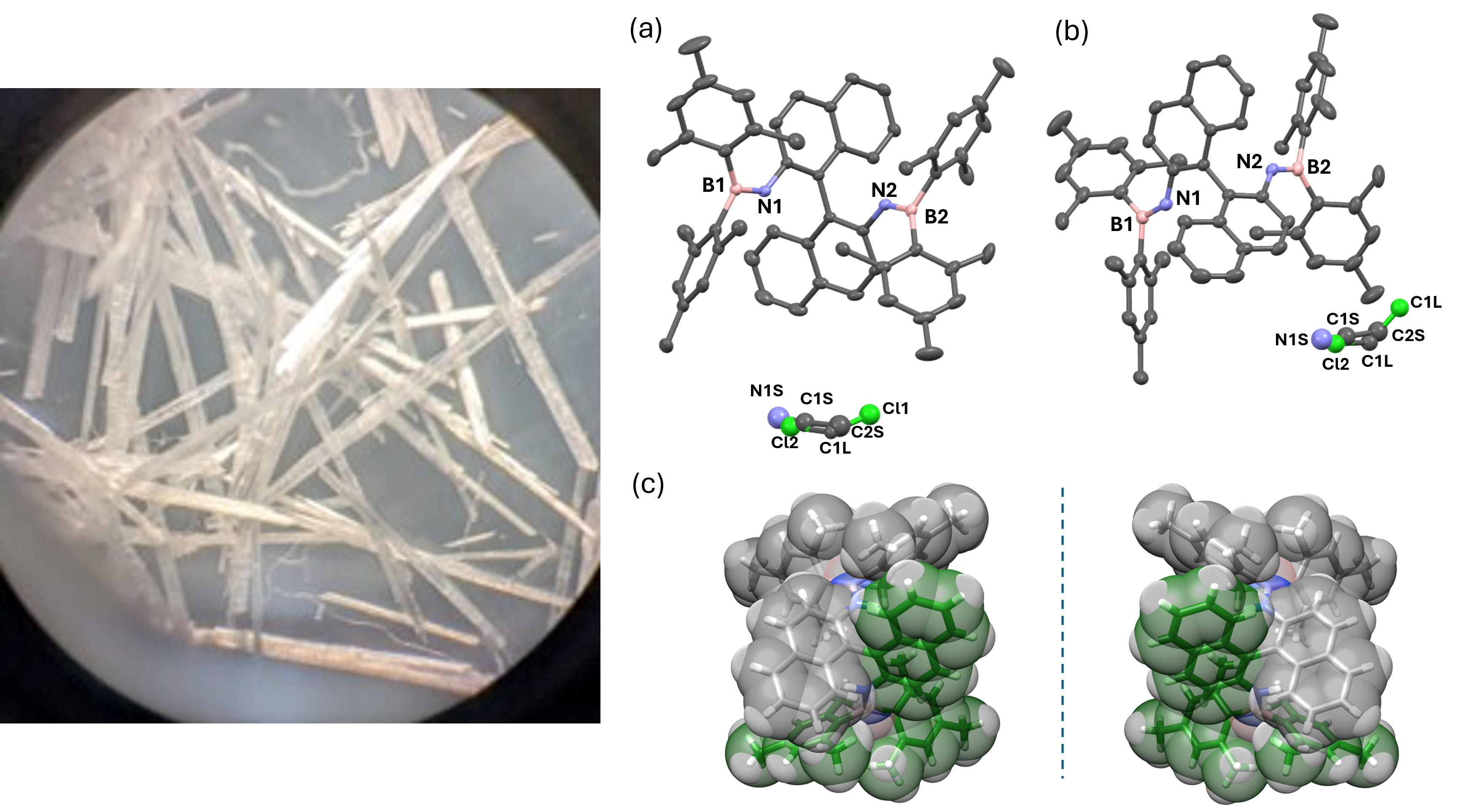 Building on these successes, the focus of the current work was to extend the explorations in these unique systems to unlock even more complex photophysical properties. We envisioned that introducing an atropisomeric axis between two aminoborane units would structurally lock the system. This molecular rigidification could suppress non-radiative decay due to molecular motion, promote intersystem crossing (ISC) via enhanced spin–orbit coupling, and induce chirophosphorescence activity. The synthesis involved the formation of B=N bonds, which are known to be sensitive to moisture and oxygen. The reaction also required stringent conditions, including pyrophoric n-butyllithium additions in a −78 °C bath. Initially, yields were low and inconsistent. After methodical optimization, particularly of reagent stoichiometry, we improved the outcomes considerably. We observed needle-shaped crystals from pure fractions collected after silica gel column chromatography (Figure 1). However, the crystals reverted to powdery solids soon after solvent evaporation. This led us to conjecture that the presence of solvent molecules is necessary to stabilize the crystal lattice of these systems. However, we resolved this issue in collaboration with Prof. Neal Hickey at the University of Trieste, Italy. With the right combination of solvents, we successfully obtained diffractable-quality crystals and confirmed the structures. As expected, the axially locked aminoboranes exhibited p-RTP and circularly polarised luminescence (CPL). The enhanced pRTP and CPL characteristics in aminoboranes can be attributed to the collaborative effects of axial chirality and BN-imposed spin-orbit coupling. The glittering and afterglow properties of these compounds were utilized in anti-counterfeiting applications.
Building on these successes, the focus of the current work was to extend the explorations in these unique systems to unlock even more complex photophysical properties. We envisioned that introducing an atropisomeric axis between two aminoborane units would structurally lock the system. This molecular rigidification could suppress non-radiative decay due to molecular motion, promote intersystem crossing (ISC) via enhanced spin–orbit coupling, and induce chirophosphorescence activity. The synthesis involved the formation of B=N bonds, which are known to be sensitive to moisture and oxygen. The reaction also required stringent conditions, including pyrophoric n-butyllithium additions in a −78 °C bath. Initially, yields were low and inconsistent. After methodical optimization, particularly of reagent stoichiometry, we improved the outcomes considerably. We observed needle-shaped crystals from pure fractions collected after silica gel column chromatography (Figure 1). However, the crystals reverted to powdery solids soon after solvent evaporation. This led us to conjecture that the presence of solvent molecules is necessary to stabilize the crystal lattice of these systems. However, we resolved this issue in collaboration with Prof. Neal Hickey at the University of Trieste, Italy. With the right combination of solvents, we successfully obtained diffractable-quality crystals and confirmed the structures. As expected, the axially locked aminoboranes exhibited p-RTP and circularly polarised luminescence (CPL). The enhanced pRTP and CPL characteristics in aminoboranes can be attributed to the collaborative effects of axial chirality and BN-imposed spin-orbit coupling. The glittering and afterglow properties of these compounds were utilized in anti-counterfeiting applications.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in