Why analyse the sugars of a nematode worm? In this case, our quest has its starting point in vaccine development. In many parts of the world, dog owners annually administer a chemical prophylaxis to their pets against the infection with Dirofilaria immitis, more commonly known as dog's heartworm. However, sometimes there is resistance to the drug and also climate change means that the geographical range of the worm and its intermediate host (commonly Aedes aegypti, also familiar as a vector for Zika and Dengue viruses) is increasing. Therefore, vaccination is being considered as an approach to control dirofilariasis - but, as recombinant proteins have proven to be poor in trials to combat other nematodes, while the natural glycoproteins are preferred, the post-translational modifications of worm glycoproteins have taken centre stage in such approaches.
There is also another aspect to sugars - particularly how they modulate the interaction between parasites and their hosts. After transmission via the mosquito, D. immitis takes up residence in the heart cavities of the infected animals and there it stays for years, miraculously remaining "hidden" or untouched by the dogs' immune system. Thus, there have to be molecules turning down or deflecting the host immune response - and complex carbohydrates or glycans are key candidates. Glycans are a common feature on proteins and have a mind-boggling variety of biological functions; when attached via asparagine (N) residues, they are named N-glycans, which in parasites are very diverse in structure and often quite different to the ones in mammals. However, when we started there was relatively little information on the structure of the N-linked glycans of D. immitis and their role in the interaction with the dog's immune system.
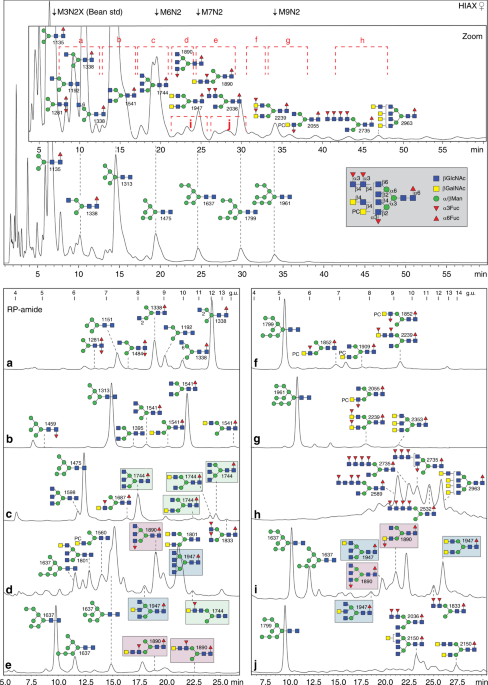
Investigating the N-glycans of D. immitis seemed a daunting task: the starting worm material is rare, as it comes from worms surgically removed from infected dogs and N-glycan analysis is intrinsically complicated by their isomeric and branched nature. Luckily, complex N-glycan structures and small organisms are the favorite topics of the Molecular Glycobiology group at the Universität für Bodenkultur in Vienna. Over the years, this group has specialized in in-depth N-glycan analysis of organisms such as mollusks, insects, protozoans, and nematodes using a combination of chromatographic, biochemical, and MALDI mass spectrometric techniques. In order to work with them I planned a three months visit to Vienna where, under the guidance of Katharina Paschinger and Iain Wilson, I embarked on the analysis of D. immitis N-glycans as part of my thesis funded via the Glycopar Marie Curie Initial Training Network. This proved to be a complicated journey full of surprises, so much that the original three months in Vienna were followed by other two visits, but well worth the effort. We realized early on that these N-glycans were extremely diverse, complex in structure, and bigger than the average worm N-glycans, some containing up to more than 30 monosaccharide units and reaching 7000 Da of mass. As reported in our Nature Communications paper, these big structures are composed of long chains of N-acetylhexosamine residues decorated with phosphorylcholine, α1,3-fucose, and an initially mysterious anionic component. The latter proved to be glucuronic acid, a striking feature previously not found on nematode N-glycans.
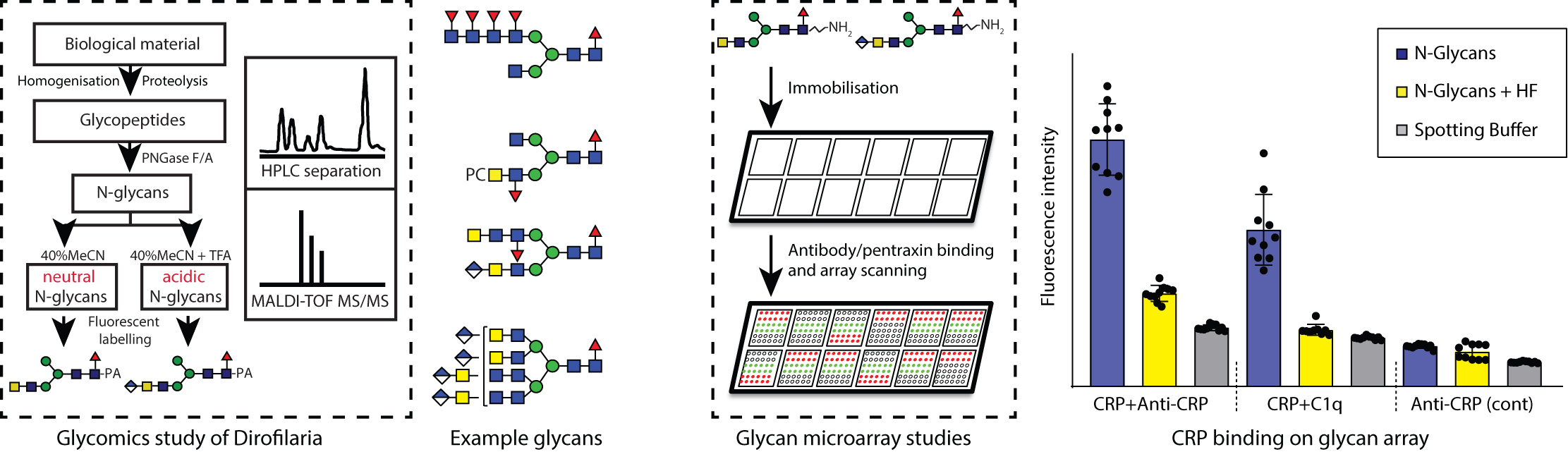
Some of the motifs found in D. immitis N-glycans have been shown to have immunomodulatory activity in other parasites and indeed some people swallow eggs of some worms to overcome allergy or autoimmune diseases. To investigate if such motifs actually interact with elements of the mammalian immune system, we used native glycan microarrays, which needed to be tailor-made by Barbara Eckmair and Shi Yan. They spotted the parasite’s own N-glycans onto glass slides and probed them with C-reactive protein (as CRP this may be familiar from lab tests of your own blood for inflammation), mannose-binding lectin, and infected dogs' sera. Neutral glycans are preferentially recognised by IgM in dog sera or by mannose binding lectin when antennal fucose and phosphorylcholine residues are removed; this pattern of reactivity is, however, reversed for CRP, which was in turn bound by the complement component C1q. Thereby, we showed that the N-glycans of D. immitis contain features which may either turn down immune functions in the host or enable avoidance of immune surveillance. Certainly, it will be interesting to see if future experiments can shine more light on these interactions and so more fully show how glycans of D. immitis or other worms are a sort of long-term residency permit for survival in their mammalian hosts.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Reference:
Highly modified and immunoactive N-glycans of the canine heartworm
Nature Communications 10, 75 (2019)
https://www.nature.com/articles/s41467-018-07948-7