Low levels of monkeypox virus neutralizing antibodies after MVA-BN vaccination
Published in Healthcare & Nursing
In May 2022, monkeypox (MPX) was identified in several countries in which MPX cases had not been reported previously, after which MPXV rapidly spread in Europe and the United States among individuals who had not traveled to endemic areas.1 On July 23, 2022, this ongoing MPX outbreak was declared a public health emergency of international concern (PHEIC) by the Director-General of the World Health Organization (WHO).2 MPX is caused by the monkeypox virus (MPXV), which belongs to the Orthopoxvirus genus of the Poxviridae family of large double-stranded DNA viruses.
Vaccines against poxviruses had already been developed in the 1960s and 1970s to assist with the eradication of smallpox. The 1st and 2nd generation smallpox vaccines contained infectious vaccinia virus (VACV) grown either in the skin of live animals (e.g., Dryvax), the chorioallantoic membrane of eggs (e.g., VACV-Elstree), or cell culture (e.g., ACAM2000).3 The 3rd generation of smallpox vaccines was based on an even further attenuated VACV obtained by serial passage in chicken embryo fibroblasts (CEF), known as modified vaccinia virus Ankara (MVA). A study in the Democratic Republic of the Congo suggested that smallpox vaccines were also effective against MPX to a certain extent, and MVA vaccination was shown to prevent severe MPX in non-human primates.4-7 However, there is no efficacy and immunogenicity data of the 3rd generation MVA smallpox vaccine against MPX in humans. Despite this lack of efficacy data against MPX or demonstrable MPXV-neutralizing antibodies in vaccinated individuals, MVA-BN was licensed as a vaccine against MPX in humans in Canada (known as Imvamune) and the United States (known as Jynneos), and was recently approved by the European Medicines Agency (EMA) ‘under special circumstances’ (known as Imvanex), to assist with interrupting the ongoing MPX outbreak.
The aim of our study was to address the crucial knowledge gap whether MVA-BN vaccination induces MPXV-reactive and neutralizing antibodies. To answer that question, we employed a poxvirus-specific ELISA to detect poxvirus-binding antibodies, and developed a plaque reduction test to measure antibodies that can specifically neutralize MPXV. These assays were validated with sera from individuals vaccinated prior to the eradication of smallpox with the historic vaccine (prior to 1974 in the Netherlands), and sera obtained from MPX patients. We had access to four distinct serum panels: (1) an age-panel of 126 sera obtained from individuals born prior to or after 1974, (2) a diagnostic panel of 72 sera obtained from MPXV PCR-negative and -positive individuals, (3) an Imvanex vaccination panel of 56 sera obtained pre- and post-vaccination, and (4) an MVA-based influenza vaccination panel of 86 sera obtained pre- and post-vaccination.8
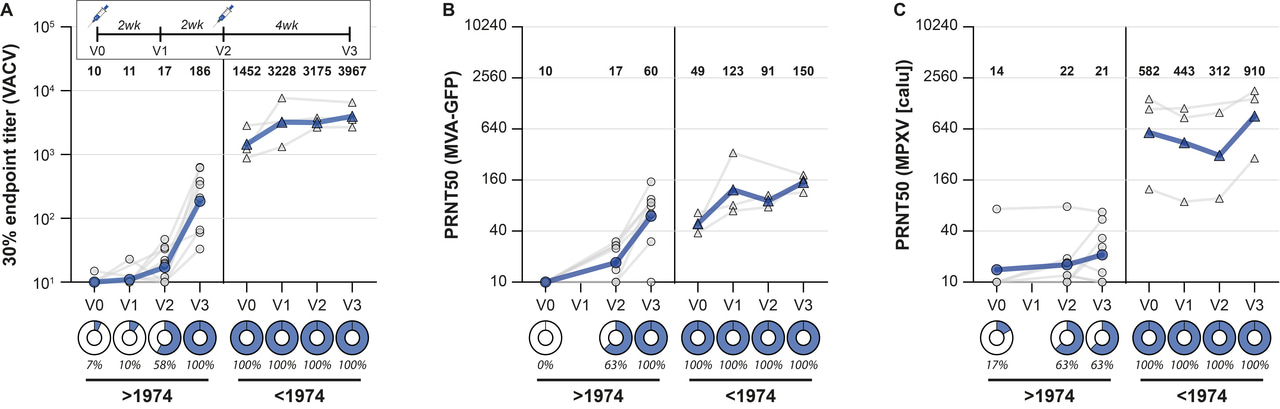
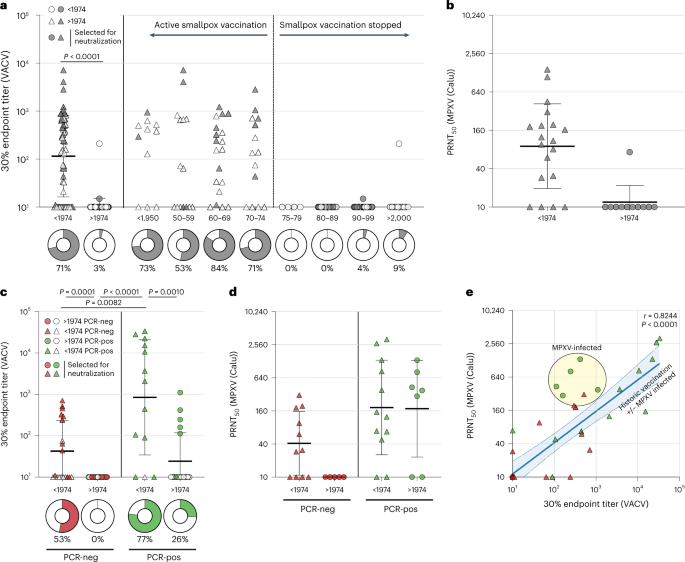
In the age-panel, we confirmed that our ELISA could detect poxvirus-specific binding antibodies in individuals born prior to 1974 (inferred to be vaccinated against smallpox), but not in individuals born after 1974 (inferred not to be vaccinated against smallpox) (Figure 1A). Antibodies were detected >70 years after historic smallpox vaccination. These sera could also neutralize MPXV, confirming that the historic vaccine induced MPXV-neutralizing antibodies (Figure 1B).
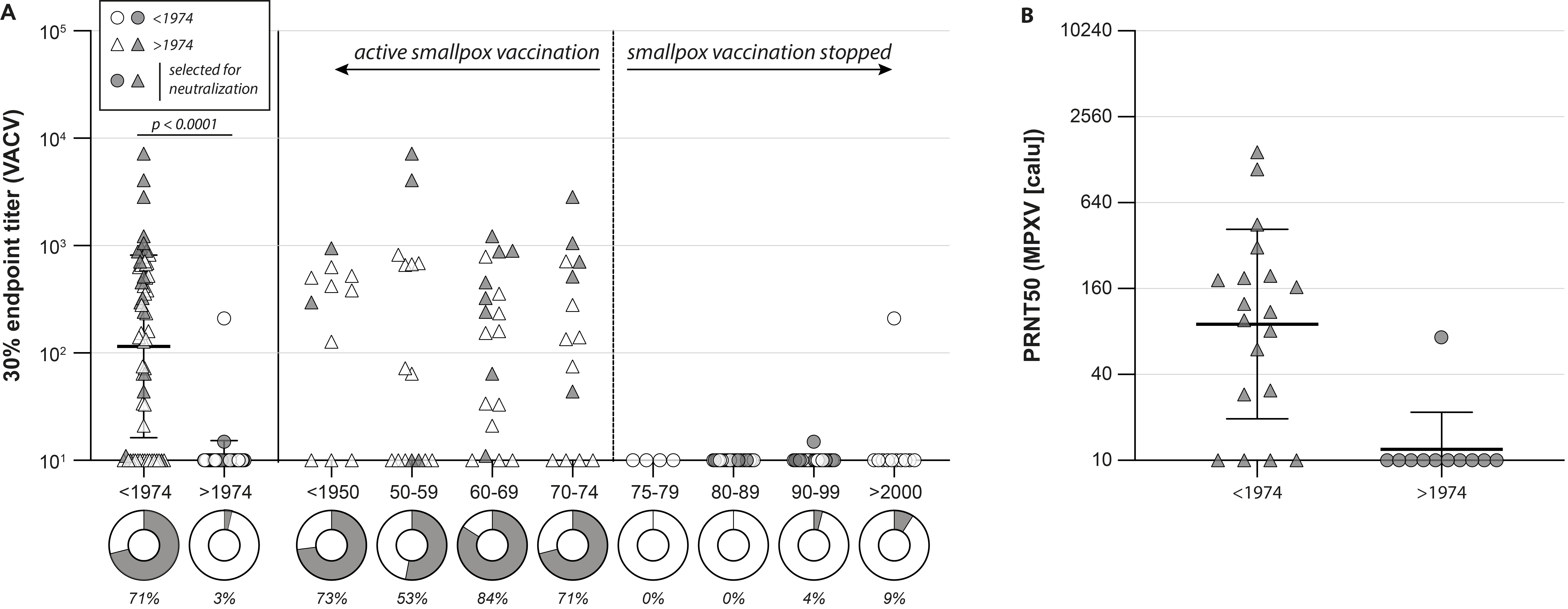
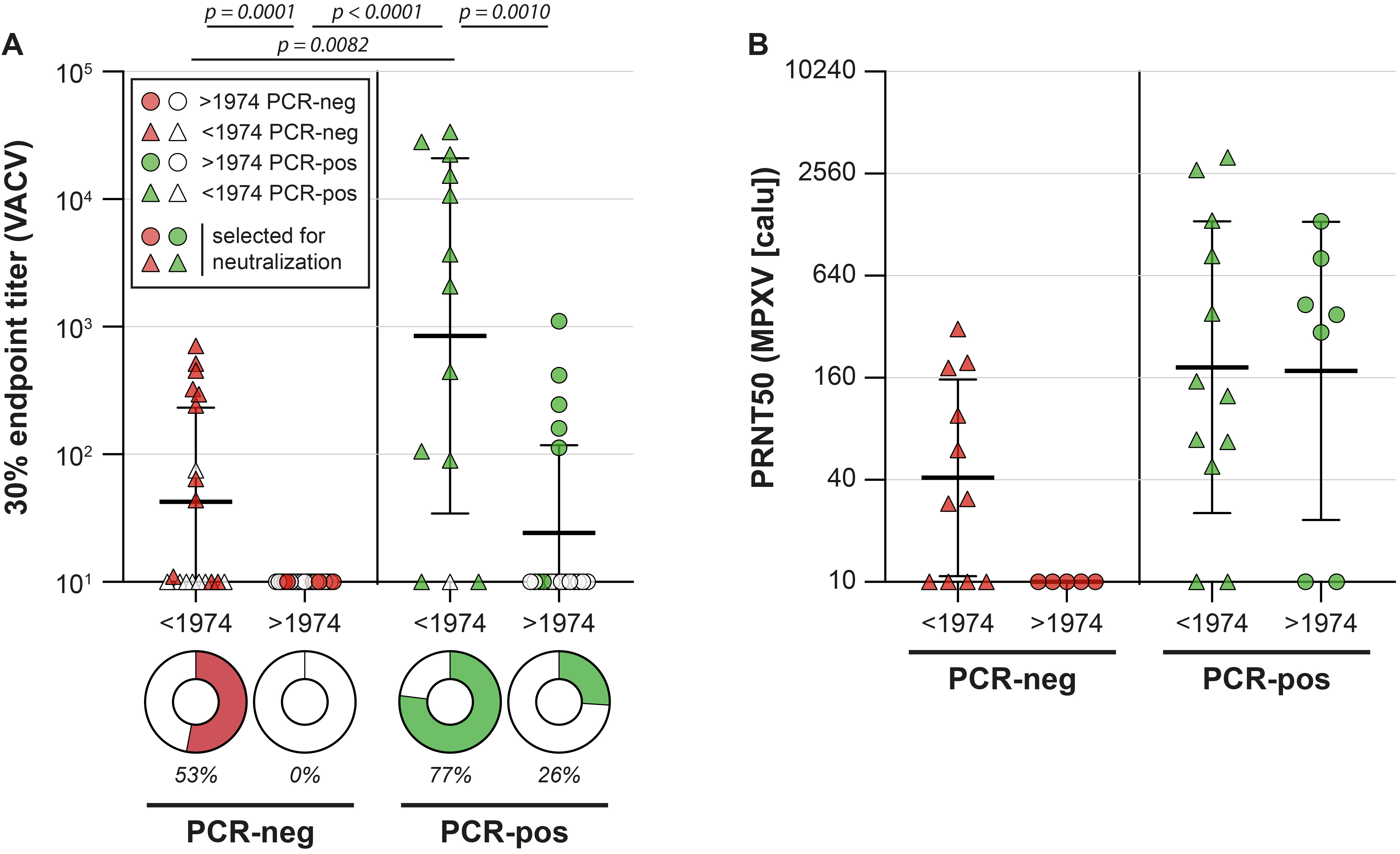
Not surprisingly, we also detected poxvirus-specific antibodies in MPXV PCR-positive individuals from the diagnostic panel, which also had neutralizing capacity. A MPXV infection in individuals born prior to 1974 led to even higher antibody levels, suggesting that the infection boosted antibodies that were previously induced by the original smallpox vaccination decades ago (Figure 2A and 2B).
To answer our specific question (whether MVA-BN vaccination induces MPXV-reactive and -neutralizing antibodies), we next measured poxvirus-binding antibody levels in MVA-BN vaccinated healthcare workers. Sera were obtained pre-vaccination, 2 and 4 weeks after the first vaccination, and four weeks after the second vaccination. As expected, poxvirus-binding antibodies were detected after MVA-BN vaccination, increasing in level over time and with additional vaccination. These sera were capable of neutralizing the vaccine virus, but, more importantly, only developed relatively low levels of antibodies that could neutralize MPXV (Figure 3A-C). We observed similarly low antibody levels against MPXV in people vaccinated with an MVA-based influenza vaccine, but also showed that a third vaccination with MVA significantly boosted MPXV-neutralizing antibodies (Figure 3D-F).
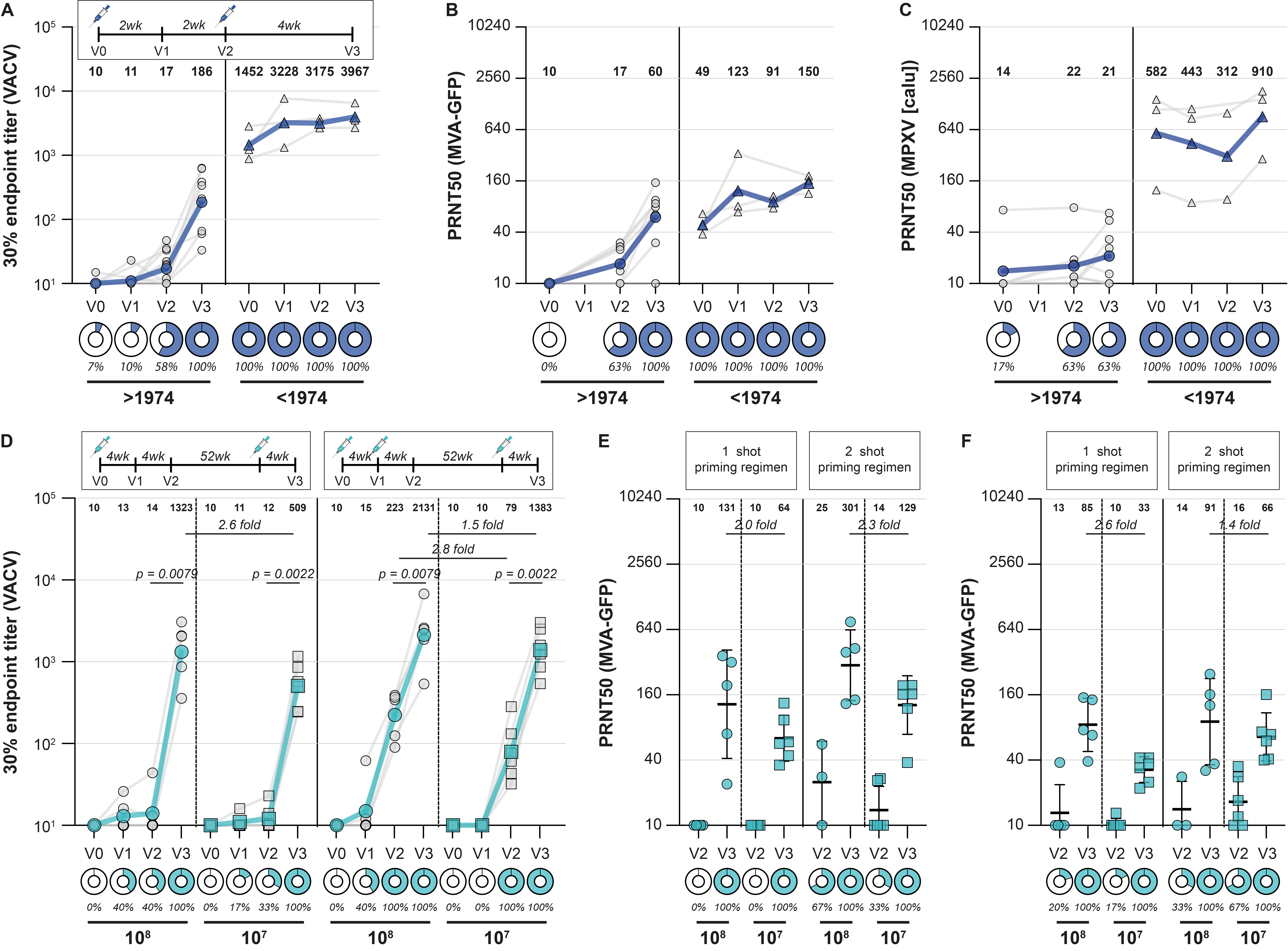
Evidence for cross-protection afforded by smallpox vaccines against MPX has been inferred from animal experiments and from observational studies conducted during the period of enhanced surveillance in the endgame of smallpox eradication.4-7 In those studies, partial clinical protection was observed, but data on levels of MPXV-neutralizing antibodies were lacking. We now show that the primary MVA immunization series in non-primed individuals yielded relatively low levels of neutralizing antibodies, raising the question whether vaccinated individuals are now protected. At this moment, it is unclear what the relatively low MPXV-neutralizing titers mean for protection against disease, severity of symptoms, and transmissibility, and what the role of other correlates of protection (such as virus-specific T-cell responses) could be. Cohort studies following vaccinated individuals are necessary to further assess vaccine efficacy in risk populations and determine correlates of protection for this emerging pathogen.
References
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med 2022;387:679-91.
- Wenham C, Eccleston-Turner M. Monkeypox as a PHEIC: implications for global health governance. Lancet 2022.
- Paran N, Sutter G. Smallpox vaccines: New formulations and revised strategies for vaccination. Hum Vaccin 2009;5:824-31.
- Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004;428:182-5.
- Earl PL, Americo JL, Wyatt LS, et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci U S A 2008;105:10889-94.
- Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ 1988;66:465-70.
- Stittelaar KJ, van Amerongen G, Kondova I, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol 2005;79:7845-51.
- Kreijtz JH, Goeijenbier M, Moesker FM, et al. Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect Dis 2014;14:1196-207.
Follow the Topic
-
Nature Medicine

This journal encompasses original research ranging from new concepts in human biology and disease pathogenesis to new therapeutic modalities and drug development, to all phases of clinical work, as well as innovative technologies aimed at improving human health.
Related Collections
With Collections, you can get published faster and increase your visibility.
Stem cell-derived therapies
Publishing Model: Hybrid
Deadline: Mar 26, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in