Real-time multimodal phenotyping reveals distinct tumor cell dynamics and immune escape mechanisms in T cell therapies
Published in Bioengineering & Biotechnology
Adoptive T cell transfer therapy (ACT) is an advanced approach that uses the cells of patients’ immune system to eliminate cancer, there are tumor-infiltrating lymphocytes (TIL) therapy, chimeric antigen receptor T cell (CAR-T) therapy, etc. Although current ACT has demonstrated clinical potential against types of cancer, therapeutic efficacy and applicability are still problems, and the mechanisms are unclear. The ultimate success of these therapies (i.e., the precise failure or success of T cell-mediated cytotoxicity) is determined by intricate molecular and biophysical exchanges at the single-cell interface. Conventional methods, often limited to endpoint analysis or requiring disruptive sampling, fail to capture the longitudinal dynamics of critical events such as early tumor resistance, metabolic adaptation, and sophisticated immune evasion mechanisms that dictate patient outcomes. This critical demand and technical limitation motivate our research.
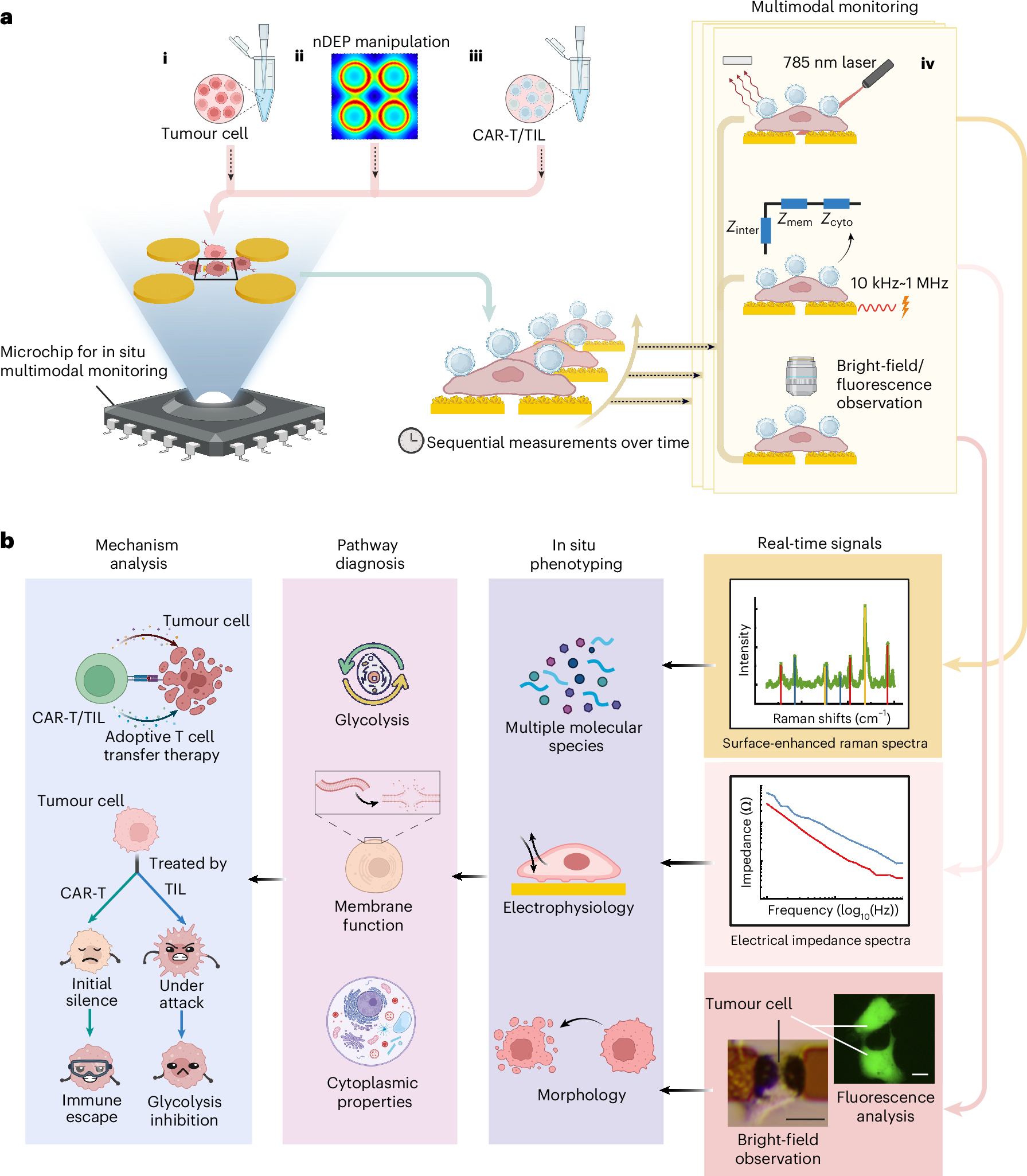
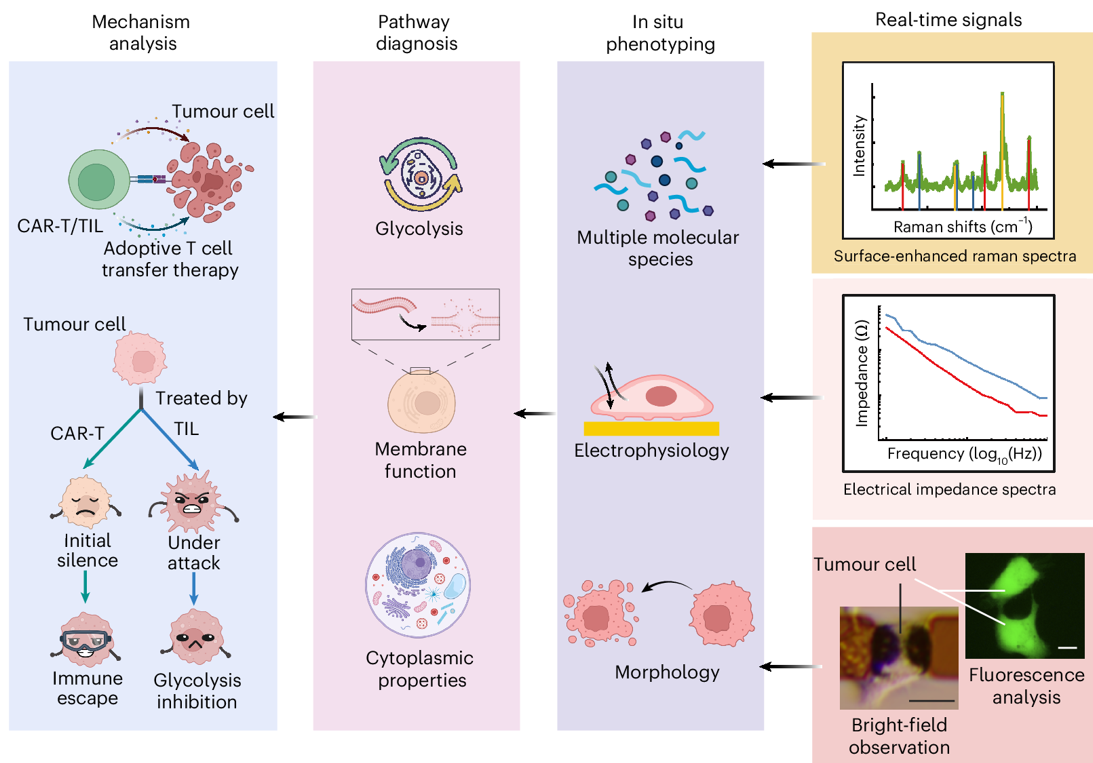
Figure. 1 | Schematic diagram of multimodal phenotyping system. a. Flow chart of multimodal phenotyping. (i) Tumor cells are seeded on a microchip. (ii)Negative dielectrophoretic manipulation is applied to trap the tumor cells onto the center probes. (iii)After cell adhesion, adoptive T cells at a ratio of T: cancer cell 4: 1 are added. (iv)Multimodal single-cell phenotyping is conducted on live tumor cells in real-time. b. Schematic of multimodal phenotypic analysis. Real-time signals of surface-enhanced Raman spectra (SERS), electrical impedance spectra (EIS), bright-field images as well as fluorescence images of single tumor cell (scale bar is 20 ) are acquired at sensing layer, from which the multiple molecular species, electrophysiological properties, and morphologies of target tumor cell are figured out. The multimodal phenotypes are utilized to diagnose the key molecular events of glycolysis, membrane and cytoplasm, further reflecting the mechanism of such as killing process and immune escape for tumor cells when attacked by ACT cells. This figure is created with BioRender.com.
In our recent work published in Nature Biomedical Engineering doi:10.1038/s41551-025-01582-7, we propose a real-time, label-free, multimodal cell phenotyping system integrating three orthogonal analytical modalities: Electrical Impedance Spectroscopy (EIS), Raman Spectroscopy, and Optical Microscopy. Our system acts as a highly sensitive observatory on a chip, enables tracking dynamic progression of live single tumor cell in terms of its electrophysiological properties, molecular profiling, and micromorphology. This synergy allows simultaneous, label-free quantification of convergent biophysical and biochemical signatures, specifically tracking tumor cell metabolic activity, membrane integrity, and cytoplasmic properties with single-cell resolution (Figure 1).
Using this real-time multimodal phenotyping system, we track the dynamic interaction and death of live tumor cells during ACT therapy, capture the key molecular events in glycolysis, membrane function, and cytoplasmic properties of tumor cells. The time-varying monitoring multimodal electro-phenotype parameters can characterize dynamic changes in cellular electrophysiological states over time (Figure 2).
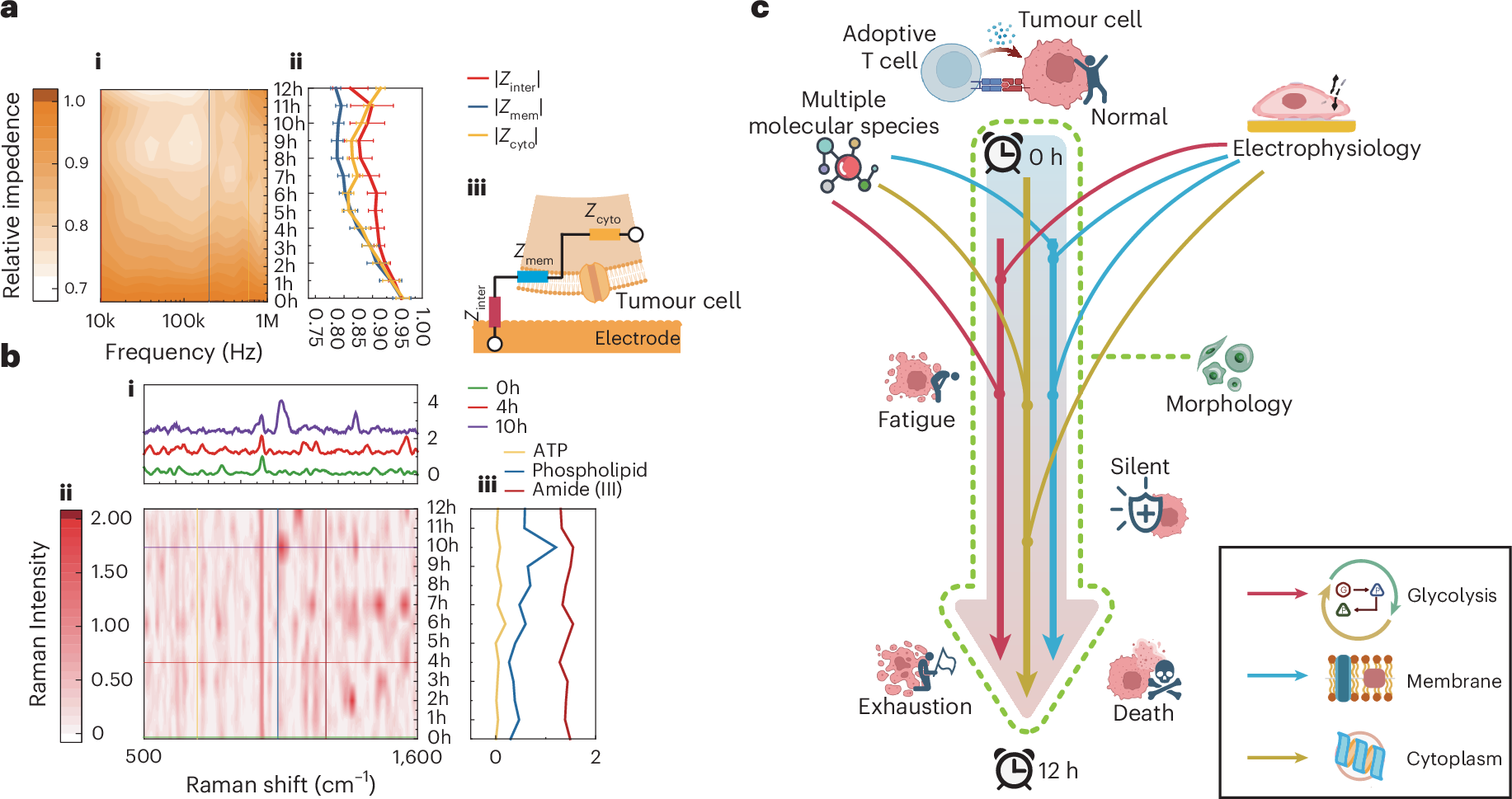
Figure. 2 | Real-time multimodal phenotyping of live single cells. a. Real-time electrical impedance spectra of single tumor cell under CAR-T treatment. (i) Normalized electrical impedance spectra contour profile. The color bar represents normalized intensity in arbitrary units (a.u.). (ii) The time-varying single-cellular impedance signals characterizing key cellular electrophysiological status. (iii) Schematic of equivalent circuit model of adherent single cell measured by microelectrodes. b. Real-time Raman spectra of single tumor cell under TIL treatment. (i) Raman spectra at the therapy time points (0h, 4h, 10h). (ii) Normalized Raman spectra contour profile. (iii) The time-varying Raman signals characterizing typical molecules (ATP, phospholipid, amide (III)). c. Schematic of dynamic tumor cytopathology monitoring through multimodal phenotyping during ACT therapy. Elements in Figures 2a and 2c are created with BioRender.com.
Our investigation reveals a striking mechanistic divergence in the cytotoxic kinetics of TIL versus CAR-T cells (Figure 3). The study finds that TILs initiate a rapid and potent metabolic siege within the first hours of interaction. Real-time tracking confirms that TILs significantly suppress the glycolytic pathway, specifically by limiting lactate production, thereby rapidly crippling the tumor cell's aggressive energy phenotype.

Figure. 3 | Dynamic divergence in Huh7 glycolysis treated by TIL and CAR-T reveal immune escape and killing mechanism. a. Flow diagram of glycolysis in Huh7. b. Flow diagram of TIL effective stages. c. Flow diagram of CAR-T effective stages. d. Dynamic molecular responses of Huh7 treated by TIL (n=11). e. Dynamic molecular responses of Huh7 treated by CAR-T (n=10). The light grey curves in Figures 3d and 3e are dynamic molecular responses of Huh7 treated by normal T cells as control group (n=14). f. Interface impedance changes of Huh7 treated by different T cells. g. Calcein-AM fluorescence staining results of Huh7 after 12h treatment, scale bar is 20 . Each representative image shows the results consistently observed in three independent experiments. Scatterplots in d–f represent individual cell samples, error bars in f were calculated from 3 consecutive measurements on the cell and represent s.d. Boxplots show cell sample statistics; boxplots in d–f show median (centre line), upper and lower quartiles (box limits) and 1.5× the interquartile range (whiskers). The line plots connect the mean values of the boxes under the same treatments. The brown arrows in d–e indicate the trend of molecular or chemical bonds changes, and the grey dashed lines in d-f represent the division of time periods.Elements in a-c are created with BioRender.com.
In contrast, the cytotoxicity mediated by CAR-T cells presents a more nuanced and clinically challenging picture. We uniquely uncover a distinct defensive phenomenon termed "Tumor Silent Escape" during the initial phase of CAR-T attack. In this critical early window, although Raman and EIS signals indicate the onset of structural damage, subsequent metabolic inhibition is markedly delayed. This temporal disconnect allows a subpopulation of tumor cells to temporarily conceal their metabolic vulnerability, thereby evading comprehensive immune elimination. It is not until the middle-to-late stages that the system observes the expected catastrophic metabolic collapse and terminal cytotoxicity, highlighting a significant and previously uncovered vulnerability in CAR-T efficacy.
The profound clinical significance of this work lies in providing an actionable "Molecular-Level Therapeutic Battle Map" for next-generation precision immunotherapy. By quantitatively defining the CAR-T-induced "Silent Escape" window and the contrasting metabolic strategies of T cell subsets, this research provides the mechanistic foundation for rational therapeutic design.
Aiming at this silent immune escape, we propose to break the silent phase at the early stage of the immune process by active intervention, such as light, heat, or electrical stimulation, which potentially inspires promising adjuvant immunotherapy. To further validate the effectiveness of intervention approach, we preliminarily adopt a light stimulation to break the “silent escape” phase of tumor cell and successfully promote the earlier killing effect.
Future work focuses on engineering advanced T cell modalities and developing combination drug protocols specifically designed to pharmacologically intervene to break this escape channel prematurely, thereby achieving sustained metabolic suppression and substantially enhancing the overall clinical efficacy and durability of CAR-T therapy. This powerful, generalized system is thus positioned as a pivotal tool to accelerate the transition toward truly personalized and mechanism-driven cancer treatment strategies.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in