Low temperature C─H and C─C activation harnessing the catalytic potential of a mixed metal nanopowder
Platinum (Pt)-group transition metal catalysts exhibit superior performance in oxidation, hydrogenation, and industrial hydrocarbon cracking processes because of their optimum binding with the substrate, ability to form diverse surface structures and allowing large coordination by the metal centers. However, the scarcity and expensive methods to synthesize appropriate catalysts for these metals limit their broad applications in reality. Consequently, there is a continuing effort to develop a next generation catalyst class consisting of earth-abundant, affordable elements.
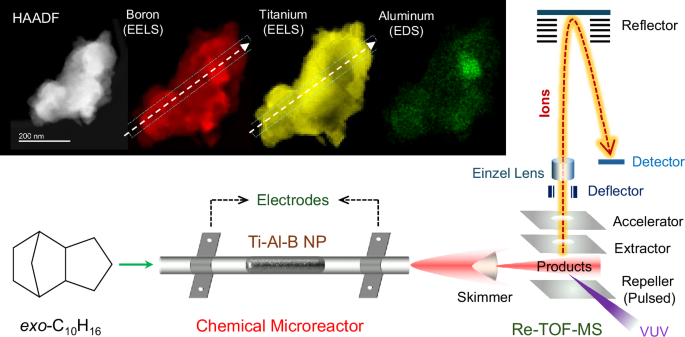
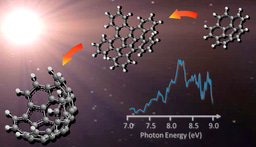
Our goal has been to unravel the key catalytic step involving fundamental reactions of the titanium-aluminum-boron nanopowder (TiAlB NP) and prototypical saturated hydrocarbon exo-C10H16, utilizing a catalytic microreactor setup to study high temperature reactions probed via a sensitive, isomer-selective, single photon vacuum ultraviolet photoionization mass spectrometry at the Beamline 9.0.2, Advanced Light Source.1-3
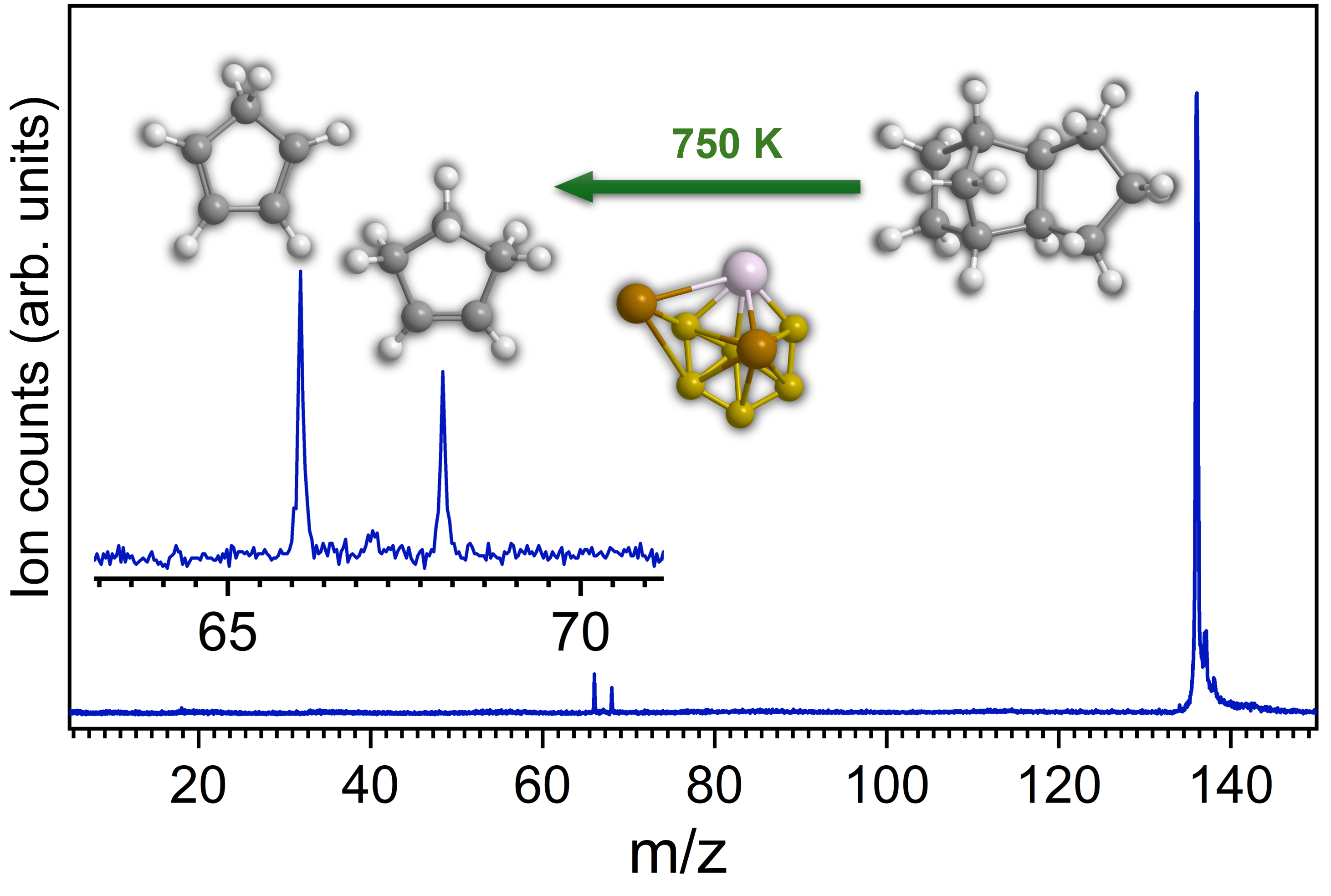
Eventually, our study revealed an initial decomposition of exo-C10H16 at 750 K yielding cyclopentadiene (C5H6) and cyclopentane (C5H8) along with molecular hydrogen (H2) in presence of TiAlB NP (Fig. 1), while the uncatalyzed pyrolysis starts at 1200 K. In addition, the full decomposition of the hydrocarbon occurred at 1050 K instead of 1600 K for the pyrolysis event. Further, utilizing a model cluster of the catalyst from a comprehensive electronic structure theory calculation, the reaction mechanism of the initial steps was unfolded, which occurs via dehydrogenation followed by retro Diels Alder reaction. The initial barrier for the C─H activation has been computed to be only 83 kJ mol-1 compared to 400 kJ mol-1 in case of uncatalyzed homolytic C─H cleavage. Each of the elements had been found to play a specific role: Ti, although insensitive to C─H activation in its metallic state, initiates the catalysis via chemisorption (‘docking’) of the hydrocarbon, adjacent B centers readily abstract hydrogen atoms and store them during the catalytic cycle, while Al stabilizes the catalyst structure yet providing space for critical docking sites for the departing hydrocarbons.
Exploiting the abovementioned state-of-the-art experimental setup, our future studies will be projected to explore diverse kinds of elemental combination in catalysts and expanding our substrates to lighter alkanes, as well as nitrogen and oxygen-containing hydrocarbons.
For more details on the experiments and results, please refer to our paper published in Nature Communications: https://doi.org/10.1038/s41467-025-62112-2.
References:
- Biswas, S. et al. Stress-Alteration Enhancement of the Reactivity of Aluminum Nanoparticles in the Catalytic Decomposition of exo-Tetrahydrodicyclopentadiene (JP-10). J. Phys. Chem. A 128, 3613-3624 (2024).
- Biswas, S. et al. Efficient Oxidative Decomposition of Jet-Fuel exo-Tetrahydrodicyclopentadiene (JP-10) by Aluminum Nanoparticles in a Catalytic Microreactor: An Online Vacuum Ultraviolet Photoionization Study. J. Phys. Chem. A 128, 1665-1684 (2024).
- Biswas, S. et al. Counterintuitive Catalytic Reactivity of the Aluminum Oxide “Passivation” Shell of Aluminum Nanoparticles Facilitating the Thermal Decomposition of exo-Tetrahydrodicyclopentadiene (JP-10). J. Phys. Chem. Lett. 14, 9341-9350 (2023).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in