Magnetically boosted 1D photoactive microswarm for COVID-19 face mask disruption
Published in Materials

Pandemic pollution caused by disposable face masks
Throughout the past COVID-19 pandemic era, face masks have become one of the most effective personal protective equipment (PPE) to protect us from the spread of the virus; but now those are scattered across the planet’s beaches, streets, and bodies of water1,2,3. Their monthly consumption is estimated to be 129 billion face masks globally, even only 1% of mismanaged disposal of them could be equivalent to tens of tons of plastic waste released to the environment4,5. Most single-use face masks, such as surgical masks or FFP-2/N95-grade respirators, are often made of synthetic plastics, thus their careless disposal poses a direct threat to wildlife as well as potential ecotoxicological effects in the form of microplastics1,3. Microplastics in waterways or oceans are eventually ingested by humans through the food chain1. Recent reports have evidenced microplastics in human blood and human placenta, raising concern for their unknown interaction in the bloodstream and translocation through the blood–brain or placental barriers6,7.
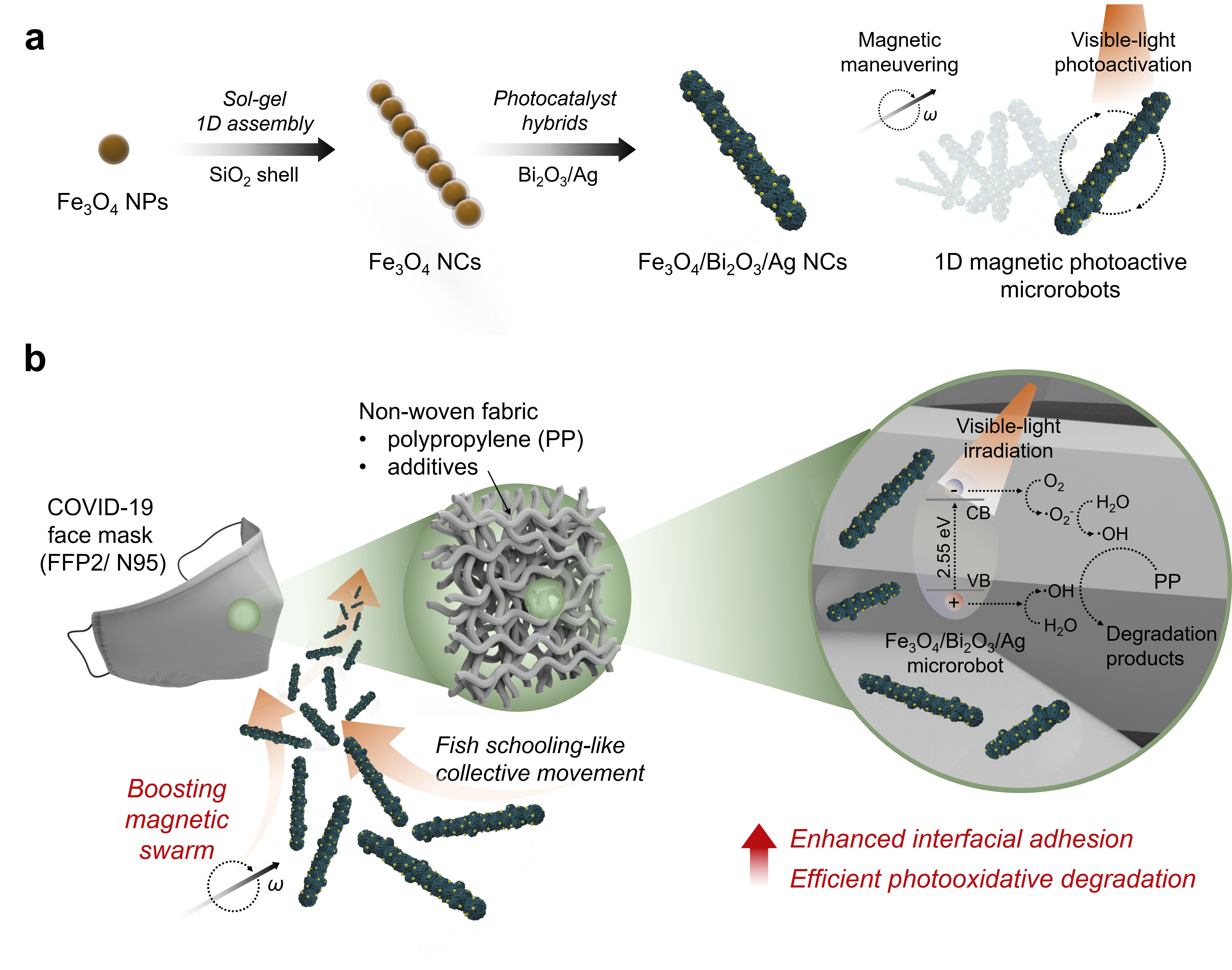
Fig. 1. Schematic illustration of a magnetically boosted 1D photoactive microswarm for accelerated COVID-19 face mask disruption. (a) Fabrication of the hierarchically hybridized 1D magnetic (Fe3O4 NC core) and photoactive (Bi2O3/Ag shell) microrobots. (b) Programmed magnetic swarm boosting for enhanced mechanical adhesion to the polypropylene microfiber network and sequential photoactivated disruption.
Microrobotic strategy to solve the COVID-19 face mask pollution
Our solution, described in this article, is to use magnetically boosted 1D photoactive microswarm that are capable of actively interacting, and accelerating the degradation of the polypropylene (PP) microfiber of COVID-19 face masks (see Fig. 1). 1D microrobots comprise an anisotropic magnetic core (Fe3O4) and photocatalytic shell (Bi2O3/Ag), which enable wireless magnetic maneuvering and visible-light photocatalysis. The actuation of a programmed rotating magnetic field triggers a fish schooling-like 1D microswarm that allows active interfacial interactions with the microfiber network. The follow-up light illumination accelerates the disruption of the polypropylene microfiber through the photo-oxidative process. The magnetically boosted 1D microswarm leads to ≈26-fold enhanced adhesion on the PP microfiber network, which is evaluated by hyperspectral dark-field microscopy. After the light irradiation, physical deformation with ≈1.9-fold increased surface area, ≈26-fold higher oxidized functional groups, weakened crystallinity, and enlarged oxidized sites were evidenced, confirming the accelerated disruption of the PP filter membranes through the photo-oxidative process.
In this article, an intriguing microrobotic strategy is proposed to ameliorate the current pandemic-associated plastic waste pollution. An active magnetic photocatalyst microswarm system could be used to treat various post-consumer plastic wastes and other environmental pollutants.
For further information, please read our paper “Magnetically boosted 1D photoactive microswarm for COVID-19 face mask disruption” published in Nature Communications, https://doi.org/10.1038/s41467-023-36650-6.
References
- Selvaranjan, K., Navaratnam, S., Rajeev, P. & Ravintherakumaran, N. Environmental challenges induced by extensive use of face masks during COVID-19: a review and potential solutions. Environ. Chall. 3, 100039 (2021).
- Silva, A. L. P. et al. Risks of COVID-19 face masks to wildlife: present and future research needs. Sci. Total Environ. 792, 148505 (2021).
- Deng, W. et al. Masks for COVID‐19. Adv. Sci. 9, 2102189 (2022).
- Prata, J. C., Silva, A. L., Walker, T. R., Duarte, A. C. & Rocha-Santos, T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 54, 7760–7765 (2020).
- Kwak, J. I. & An, Y. Post COVID-19 pandemic: Biofragmentation and soil ecotoxicological effects of microplastics derived from face masks. J. Hazard. Mater. 416, 126169 (2021).
- Leslie, H. A. et al. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 163, 107199 (2022).
- Ragusa, A. et al. Plasticenta: first evidence of microplastics in human placenta. Environ. Int. 146, 106274 (2021).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in