Metalloregulation of the essential WalK histidine kinase - sometimes you just get lucky
Published in Microbiology
Two component systems (TCS) are an important stimulus perception mechanism allowing bacteria to sense and respond to changes in the environment. TCS are usually found as pairs on the chromosome with a membrane bound or soluble histidine kinase and a DNA-binding response regulator. Recently, it was elegantly shown by Villanueva et al. (https://www.nature.com/articles/s41467-018-02949-y) that 15 out of the 16 TCS of Staphylococcus aureus could be sequentially deleted and still yield a “healthy” cell. You might ask what about the 16th, why couldn’t this be deleted? Good question and we are still coming to grips with the reason for the essentiality of the WalRK TCS (formally known as YycFG). WalRK has a direct role in the regulation of peptidoglycan hydrolases and therefore is involved in cell wall remodelling and division. Our lab became interested in WalRK following the identification of mutations that impair the activity of WalR or WalK, leading to decreased susceptibility to the last line antibiotic vancomycin and clinical treatment failure. We and others have observed that when conducting targeted mutagenesis on WalRK, often secondary mutations in genes regulated by the system can arise (https://www.nature.com/articles/ncomms14403). While frustrating and often discarded, these supressor mutations can be informative to the regulation of the system. Our work here stemmed from the isolation of one such mutation.
 Sectored colony of the Staphylococcus aureus ∆yycHI mutant.
Sectored colony of the Staphylococcus aureus ∆yycHI mutant.We observed that when you delete yycH and yycI (positive accessory regulators of WalK), genetic instability was observed, with this visualised as colony sectoring in a proportion of the population. When these outgrowths were purified, they yielded suppressor mutants, with some mapping to either WalR or WalK. We surmised that these mutations would enhance WalRK activity - something that is not seen clinically. To further characterise, one mutation in WalK was chosen to be recombined back into the wild type background (yielding WalKH271Y) and the mutation complemented on the chromosome. When analysing an essential system, the ability to investigate the mutations responsible in their native genomic context is important as gene dosage can impact regulation. You can read the paper, describing the details of what we found about the WalKH271Y mutant here (https://www.nature.com/articles/s41467-019-10932-4).
For those of you still reading on, the brief rundown on the phenotypic characterisation of the WalKH271Y mutant - it did enhance WalRK function. It lead to the loss of a lag growth phase, increased hemolysis (mediated by the SaeRS TCS), increased activity of Atl (a WalR regulated autolysin) and sensitivity to vancomycin and lysostaphin, the latter another bacterial cell-wall active agent.
But we still didn’t have any idea behind the mechanism. Linking with structural biologists in the group of Glenn King (University of Queensland) and Megan Maher (Latrobe University) moved the project forward dramatically. They crystalised the intracellular Per-Arnt Sim (PAS) domain where the mutation was centered and observed a zinc atom tetrahedrally coordinated, with H271 one of the four residues. Further biochemical experiments showed that the H271 residue played an essential role in zinc coordination. Metal binding to an intracellular PAS domain, to our knowledge, has not been reported before. While we could see a potential mechanism for WalK regulation, two chance meetings at BacPath 2015 (Chris McDevitt/Stephanie Neville) and 3rd International Conference Pathophysiology of Staphylococci in 2016 (Mike Gajdiss/Gabriele Bierbaum) led to valuable collaborations on metalloproteomics and protein phosphorylation, respectively. Their work further characterised the metal interaction which enabled us to show that metal binding in vivo repressed the kinase activity of WalK and cognate phosphorylation of WalR. Molecular modelling (Mike Kuiper, CSIRO Data61) highlighted the potential impact that metal binding plays which could be interrogated with further experimentation. Surprisingly, from protein alignments of S. aureus WalK with other homologs, suggests that metal binding might be restricted to staphylococci and enterococci.
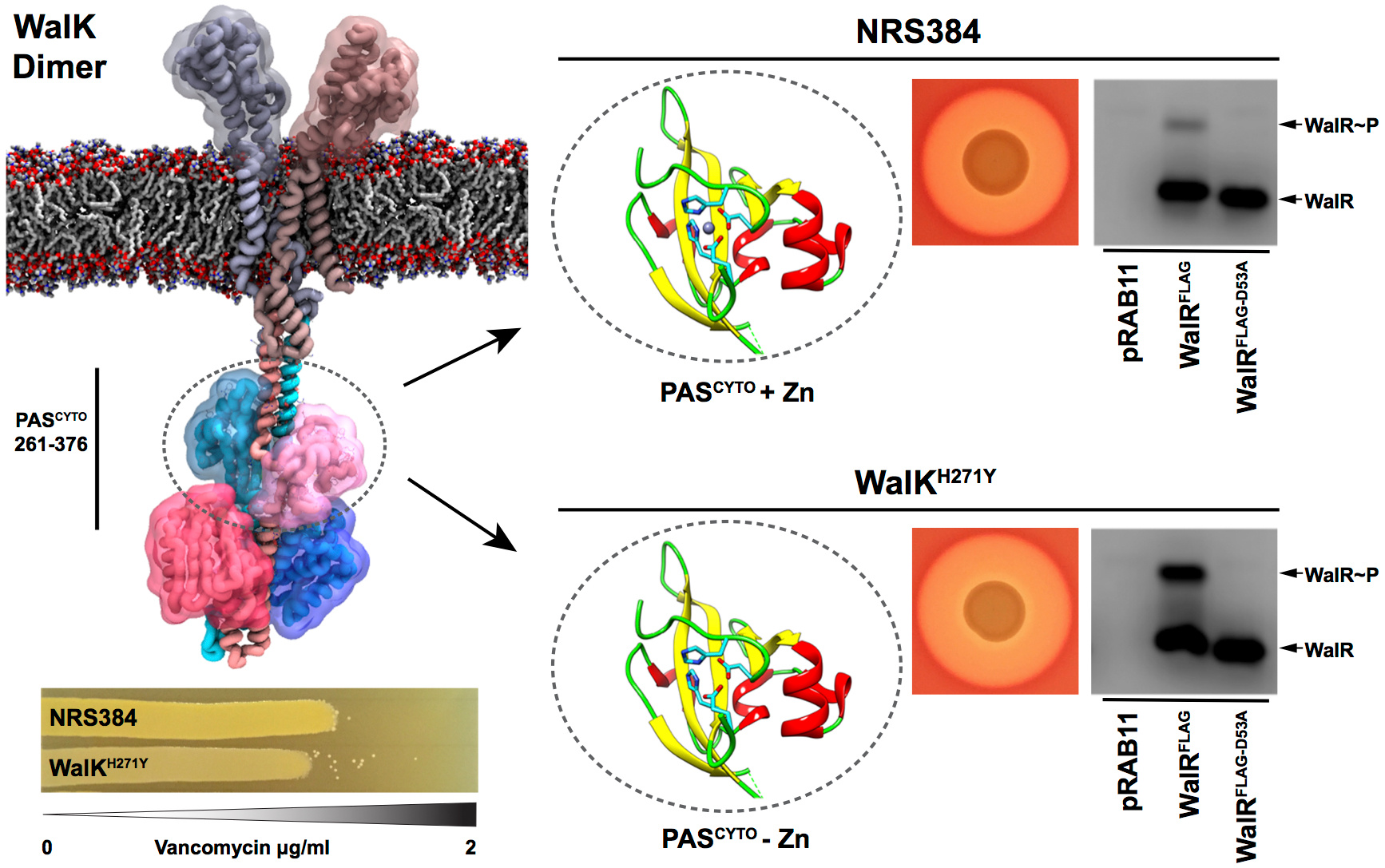
Our work suggests that zinc binding acts as a switch for WalRK, rather than playing a structural role. This work is also an experimental platform to investigate the signal(s) for the activation of WalRK and understand the biological role that metal binding plays to modulate the responsiveness of this essential sensor.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in