Behind the paper
The paper can be found here: “https://rdcu.be/LLUi”
In nature, bacteria reside in multicellular communities called biofilms, where millions of bacteria communicate, cooperate and divide labor to make a sustainable colony. Biofilms can be beneficial to other organisms, e.g., biofilms of soil bacteria formed on plant roots can prevent the growth of bacterial and fungal pathogens. In other situations, biofilms can have deleterious effects - in a clinical context, biofilms are inherently resistant to antimicrobial agents, and are at the core of many chronic bacterial infections. Thus, gaining insights into biofilm formation is of significant ecological, technological and clinical importance.
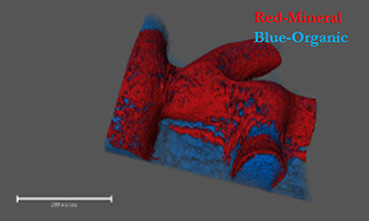
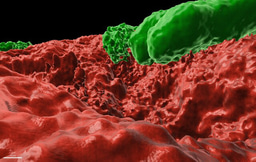
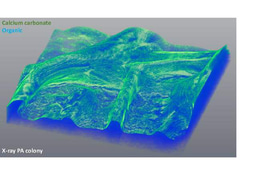
Historically, biofilms, have been thought to be held together solely by a self-produced organic extracellular matrix. Recently, we identified a novel mechanism maintaining biofilms - active production of calcite minerals (Oppenheimer-Shaanan et al., 2016). Calcite is a crystalline form of calcium carbonate, which forms exoskeletons of numerous marine multicellular organisms, among them corals. Is it possible that it might have a similar role in multicellular bacterial colonies? So far, we lacked the tools to properly image this mineral component within intact colonies. After developing a way of generating three-dimensional images of bacterial biofilms with high-resolution X-ray tomography, we discovered that as previously suggested, a biofilm is indeed a bacterial “fortress”. The calcium carbonate, it turned out, forms a well-defined crystalline scaffold, which supports the weight of the 3D structure of the colony - similarly to concrete walls of a building. Even more, it also provides a continuous “shield” of calcium mineral around the inner mass of bacterial cells.
Once we realized the potential importance of the mineralized structure, we wondered if it may have a role in protecting the cells within a biofilm from external toxins, including antibiotics. First, we showed that indeed, this mineral layer is a barrier for diffusion. This is not surprising, considering that in calcite crystals, diffusion is at least two times slower than in any organic polymer, including cellulose. Then, we tried to specifically target the mineral component and restore diffusion. When we used a small molecule to inhibit – urease - a key enzyme in biomineralization - calcium carbonate failed to form, and diffusion was restored. Targeting additional enzymes involved in the simple biochemical reactions leading to the accumulation of calcium carbonate, might be a useful approach to tackle persistent biofilm infections.
It is still to be determined how similar these mineral biofilm structures are to human bones (a crystalline form of calcium phosphate) in their development and function. How are they shaped and localized to distinct areas within the colony? What role do they play in cell-cell interactions? And finally, are they formed within a human host during chronical infection?
If you want to know more, read our brief communication in npj Biofilms and Microbiomes
Oppenheimer-Shaanan, Y., Sibony-Nevo, O., Bloom-Ackermann, Z., Suissa, R., Steinberg, N., Kartvelishvily, E., Brumfeld, V., and Kolodkin-Gal, I. (2016). Spatio-temporal assembly of functional mineral scaffolds within microbial biofilms. NPJ Biofilms Microbiomes 2, 15031.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in