Molecular motion of a nanoscopic moonlander

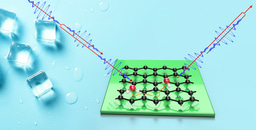
Molecular motion at surfaces determines the kinetics of processes such as heterogeneous catalysis and thin-film growth, with the diffusivity being controlled by excitation across a translational barrier. Lineshape broadening upon inelastic scattering from surfaces as illustrated in Figure 1a can be used to determine the characteristics of molecular diffusion on pico- to nanosecond timescales with low-energy neutral beams being the gentlest probing technique . Using this technique‚ we have studied the diffusion of simple molecules on 2D materials [1,2] but only recently has the approach been extended to larger and more complex molecules [3,4]
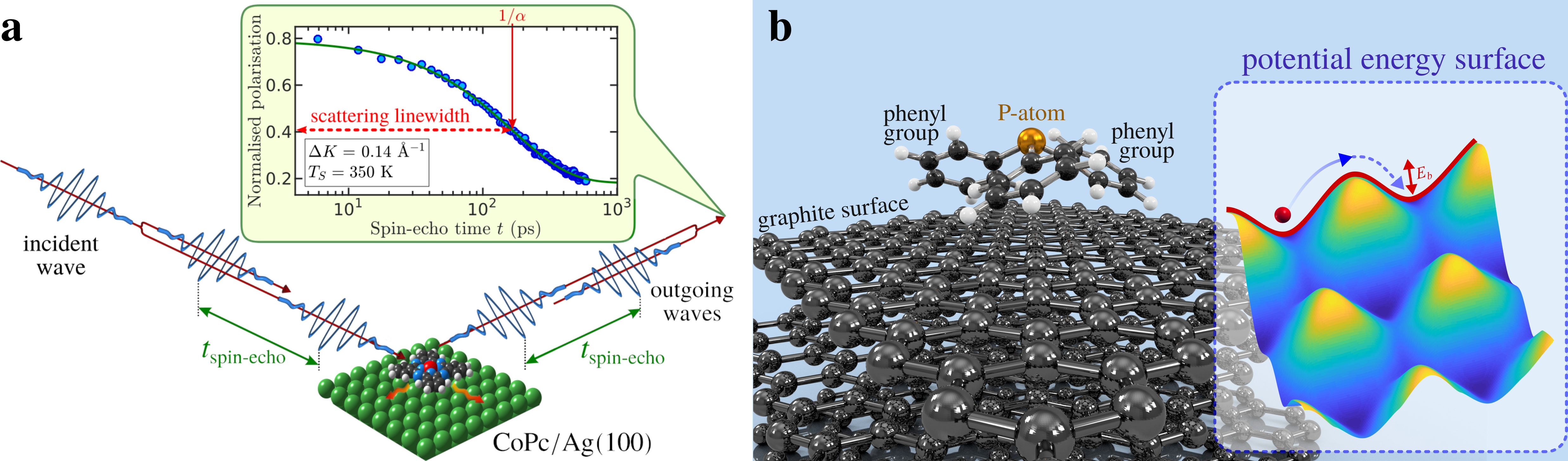
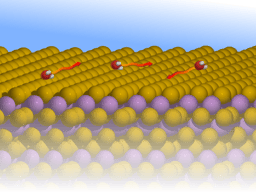
Specifically, using neutron spectroscopy, we follow the nanoscopic motion of triphenylphosphine (P(C6H5)3 or PPh3 as illustrated in Figure 1b on graphite. This "nanoscopic moonlander" follows a motion similar to that of a molecular motor: As illustrated in the video, PPh3 rolls over the surface with a fast motion of its "legs" and and a slower lateral motion.
Beyond the naïve view
In the traditional view of atomistic surface diffusion, motion is realised by a random walk of the molecule which needs to cross a barrier between two neighbouring potential wells. The molecule, often described as a point-like particle, moves by jumping from one favourable adsorption site to one of the nearest adjacent sites as illustrated in the inset on the right-hand side of Figure 1b, following the energetically most favourable route across the potential energy surface (PES). However, the current study shows that this simple picture of a point-like particle is now longer applicable to large molecules.
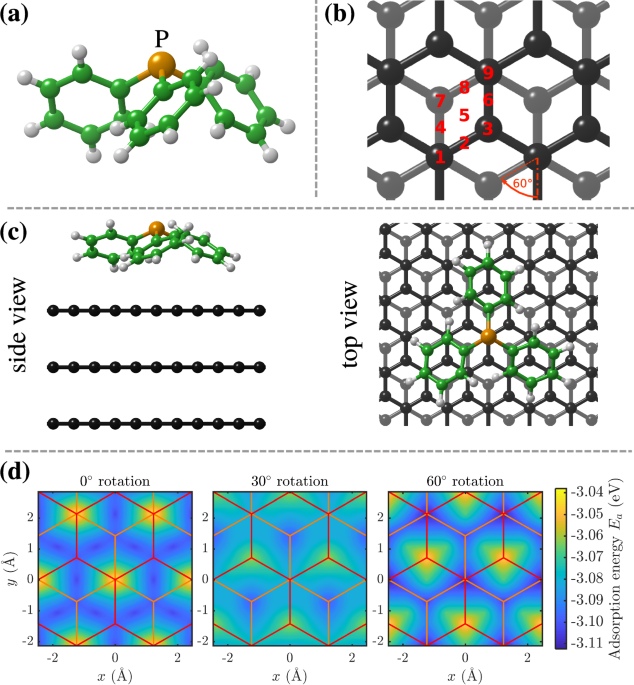
When considering larger and more complex molecules such as PPh3, the molecular degrees of freedom contribute to the motion. As illustrated in Figure 1b, PPh3 exhibits a pyramidal tripod-like structure, with the central P-atom on top, surrounded by 3 hexagonal phenyl groups. The molecule bonds to graphite with its phenyl groups pointing towards the surface in analogy to the "legs" of a nanoscopic moonlander.
The importance of internal molecular motion
As can be seen from the video below, PPh3 moves over the surface with an almost negligible activation energy for rotations and motion of the phenyl groups and a comparably small activation energy for translation. While internal molecular motions dominate at low temperatures the molecule follows an additional translational jump-motion across the surface at higher temperatures [3].
Figure 2: Video illustrating the motion of a single triphenylphosphine molecule over graphite in a top view, as extracted from a molecular dynamics simulation at a temperature of 300 K.
The unique behaviour of PPh3 is due to its three-point binding with the surface, which increases the adsorption energy compared to "flat" molecules of similar size. However, the effective diffusion barrier remains small as its "legs" in form of the phenyl group make it easier to cross the diffusion barrier [3].
Measurements across the entire temperature range
It illustrates that the molecular degrees of freedom in larger molecules are intimately connected with its diffusivity [3,4]. The latter is not only true for PPh3 with its tripod-like structure but also for large complex molecules with a planar structure such as Cobaltphtalocyanine (CoPc, as illustrated in Figure 1a where molecular rotations mean that the effective diffusion barrier becomes smaller at higher temperatures [4]. Hence, only upon exploring the full potential energy landscape benchmark data can be provided as we seek to develop models that predict the rates of chemical reactions. Both studies further highlight the importance of considering a wide temperature range to capture the complete dynamics of molecular motion on surfaces - as only at high and also technologically relevant temperatures the full complexity of the molecular motion emerges [3,4].
[1] A. Tamtögl, E. Bahn, M. Sacchi, J. Zhu, D. J. Ward, A. P. Jardine, S. J. Jenkins, P. Fouquet, J. Ellis and W. Allison, Nat. commun., 12, 3120 (2021).
[2] A. Tamtögl, M. Sacchi, I. Calvo-Almazán, M. Zbiri, M. M. Koza, W. E. Ernst, P. Fouquet, Carbon 126, 23 (2018).
[3] Tamtögl, M. Sacchi, V. Schwab, M. M. Koza, P. Fouquet. Comm. Chem., 7, 78 (2024)
[4] Sabik, J. Ellis, H. Hedgeland, D.J. Ward, A.P. Jardine, W. Allison, G. Antczak, .A. P. Jardine, W. Allison, G. Antczak, A. Tamtögl, Front. in Chem. 12, 1355350 (2024).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in