Multi-epitope spike-based antigen generate broader immune response
Published in Bioengineering & Biotechnology and Microbiology
Since the onset of the COVID-19 pandemic in late 2019, SARS-CoV-2 has undergone rapid evolution, presenting ongoing challenges to public health efforts worldwide. In comparison to many other respiratory disease-causing viruses such as Influenza, RSV; SARS-CoV-2 has shown a remarkable ability to mutate. These mutations have primarily been observed in the spike protein, a key target of the human immune system and a key component of many of the licensed vaccines. As humans developed immunity through vaccination or natural infection, new variants emerged, such as Alpha, Beta, Gamma, Delta, and the Omicron lineage. These variants have exhibited varying degrees of immune escape, making it increasingly difficult for existing vaccines to provide comprehensive protection. Compounding the problem, SARS-CoV-2 has spilled-over to other mammals like mink, cats, dogs, and deer. These cross-species infections contribute to the virus's evolution, as new mutations can emerge and be passed back to humans, potentially leading to new waves of infections.
Early in the pandemic, vaccines were developed based on the original Wuhan-1 strain of SARS-CoV-2. These vaccines proved effective initially and controlled the transmission of the disease but faced challenges as new variants, particularly Delta and Omicron, began to dominate. These two variants showed higher transmission rates and a greater ability to escape both natural and vaccine-induced immunity. In response, vaccine manufacturers have updated their formulations. Bivalent vaccines targeting both the original Wuhan strain and newer variants were introduced, but recent evidence suggests that single-antigen vaccines targeting specific Omicron sub-variants may be more effective due to the phenomenon of immune imprinting.
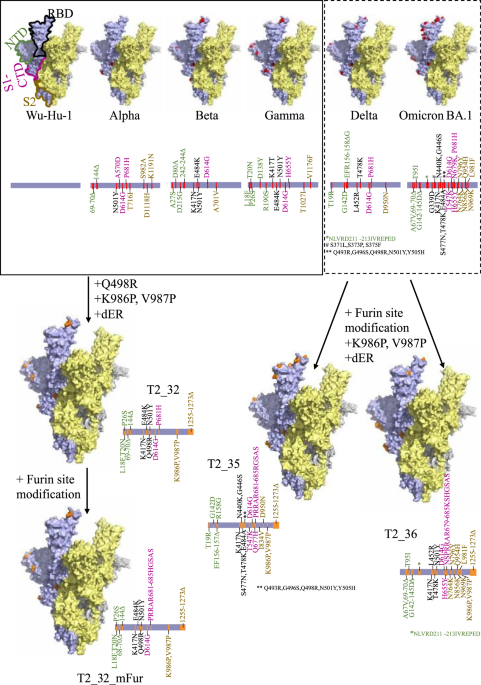
Given the rapid evolution of SARS-CoV-2, a new approach to vaccine design is necessary. Traditional strategies, which update vaccines to match the most prevalent circulating strains, may not be sufficient in an era where the virus evolves so quickly. This is analogous to the annual influenza vaccine, where effectiveness varies based on how well the vaccine matches the circulating strains. To address these challenges, Laboratory of Viral Zoonotic at University of Cambridge has explored designing next-generation vaccines that incorporate a broader range of epitopes from various SARS-CoV-2 variants. By leveraging vast repository of sequences available for SARS-CoV-2, novel spike antigens were created that include mutations observed across different variants, aiming to enhance the vaccine's ability to neutralize a wider array of SARS-CoV-2 strains. Three new spike antigens were developed:
1. T2_32: Designed prior to the global dominance of Delta and Omicron, this antigen incorporates mutations from the Alpha, Beta, Gamma, and other early variants.
2. T2_35: Combines mutations from both Delta and Omicron variants.
3. T2_36: Combines mutations from both Delta and Omicron variants.
These antigens were tested in animal models, including Guinea pigs and mice. The results showed that these new designs provided broader neutralization profiles compared to the original Wuhan-1-based and Omicron BA.1-based vaccines. Particularly, T2_32 demonstrated superior neutralization against a range of VOCs, including those that emerged after its design. Both T2_35 and T2_36 showed robust neutralizing responses against both pre-Omicron and Omicron variants, with T2_35 being particularly effective against Omicron lineages.
Our findings highlight the potential of vaccines designed with a broad range of epitopes to offer better protection against evolving SARS-CoV-2 variants compared to wild-type SARS-CoV-2. By including mutations from various VOCs, these novel spike antigens can potentially provide enhanced immunity against both current and future variants.
As the virus continues to evolve, it is crucial to adopt a proactive vaccine strategy that anticipates future changes in the virus. The traditional approach of updating vaccines based on the most recent strains may no longer be sufficient. Instead, incorporating a broader range of antigenic targets could offer a more robust and lasting protection against the ever-changing landscape of SARS-CoV-2. In conclusion, the development of multi-epitope vaccine antigens represents a promising advancement in our fight against COVID-19. By moving beyond the single strain focus and embracing a more comprehensive approach, we can better equip ourselves to handle the ongoing challenges posed by this dynamic virus.
References:
1. Carabelli, A. M. et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol 21, 162–177 (2023).
2. Tan, C. C. S. et al. Transmission of SARS-CoV-2 from humans to animals and potential host adaptation. Nat Commun 13, 2988 (2022).
3. Markov, P. V. et al. The evolution of SARS-CoV-2. Nat Rev Microbiol 21, 361–379 (2023).
4. Offit, P. A. Bivalent Covid-19 Vaccines — A Cautionary Tale. New England Journal of Medicine 388, 481–483 (2023).
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in