Multimodal Switching of a Redox-active Macrocycle
Published in Chemistry
Resorcinarene macrocycles are widely studied because they tend to self-assemble as nanocapsular structures when isolated in the appropriate isomeric form. That work has been facilitated by the availability of the macrocycle in large quantities from high-yielding one-pot multi-gram reactions. While host-guest interactions have been widely studied for these systems, we felt that the essential chemistry of the resorcinarenes had more to offer based on the introduction of functionality beyond simple aryl or alkyl substituents, and so we commenced our study by applying a common research theme in our laboratories: introduction of redox-active moieties to chromophore molecules. Hence, we introduced phenolic substituents (2,6-di-t-butylphenol, butylated-hydroxytoluene (BHT) groups), which are capable of existing in phenolic, quinonoid and even radical states (J. Am. Chem. Soc. 133, 16119–16126, 2011). To date, we have exploited these substituents mostly in the context of simple porphyrin molecules with a focus on sensing applications (Acc. Chem. Res. 48, 521–529, 2015; Chem. Commun. 48, 3951–3953, 2012) but with further emphasis on discovering switching mechanisms based on the variation of oxidation state in the molecules as is known for the corresponding porphyrin derivative.
Resorcinarene meso-substituted with BHT groups was prepared and we were subsequently excited to discover that it could indeed be isolated in three different oxidation states. Most importantly, oxidation steps can be performed independently either by exposure to light or chemically. Actually, photochemically induced oxidation had caused difficulties in the identification of the compounds even with all compounds being X-ray crystallographically characterized. While the forward oxidation steps proved reversible, it was not until we observed the compounds under acidic conditions that the final piece of the puzzle for our switching manifold fell into place. Higher oxidation states of the molecule exhibit intense colors in the presence of acids in solution and solid states. These states could also be accessed by performing oxidation steps under acidic conditions. The whole process, related electronic absorption spectra and the colours of the different switching nodes are shown in Fig. 1. We could eventually assign the intensely colored species as intramolecular charge transfer (C-T) states whose formation is promoted by protonation of quinonoid oxygen atoms. These C-T states are thought also to be stabilized by hydrogen bonding involving the acidic reagent.
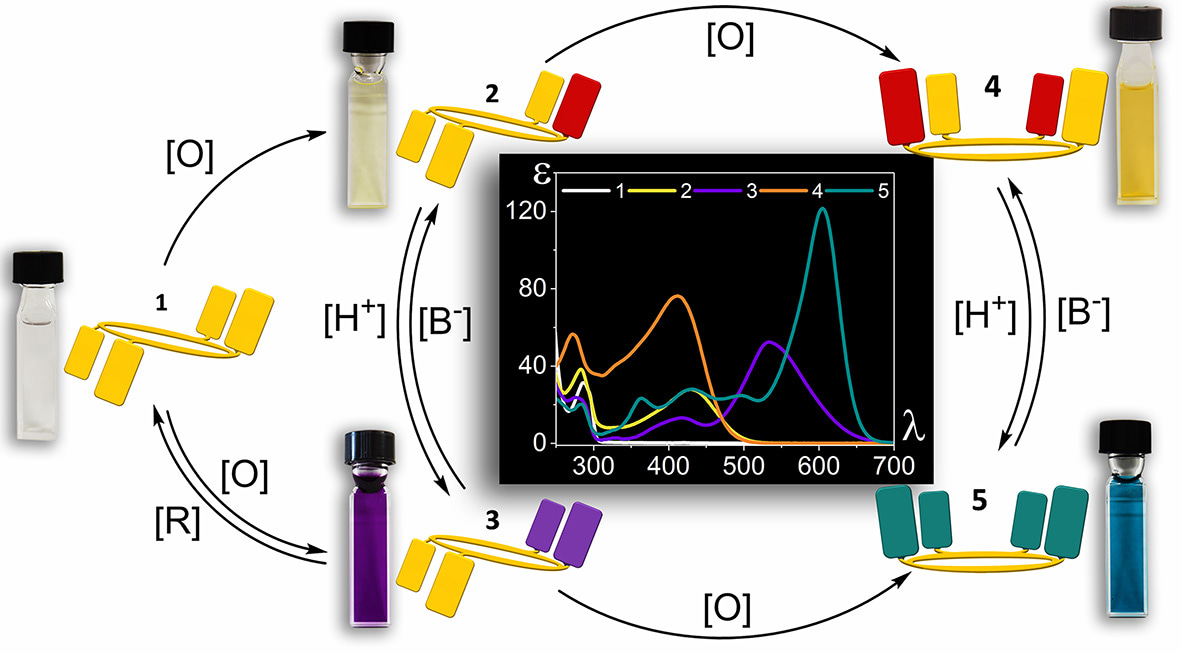
Fig. 1. Stable states of the resorcinarene molecular switch. 1: starting state (neutral, not oxidized), 2: mono-oxidized (mono-quinonoid), 4: doubly oxidized (bis-quinonoid). 3 and 5 are the acid stabilized charge transfer states of 2 and 4, respectively. Central panel shows the electronic absorption spectra of each state.
Overall, this molecular switching work illustrates that, as we suspected, there are other aspects of the resorcinarene macrocycles that deserve further investigation. These are complementary to the already fantastic array of supramolecular systems that have been reported for the resorcinarenes. We also expect that synergies will come about between the existing nanocapsular research area and emerging properties of this important class of macrocycle.
For more information please see: D. T. Payne et al. "Multimodal Switching of a Redox-active Macrocycle"
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in