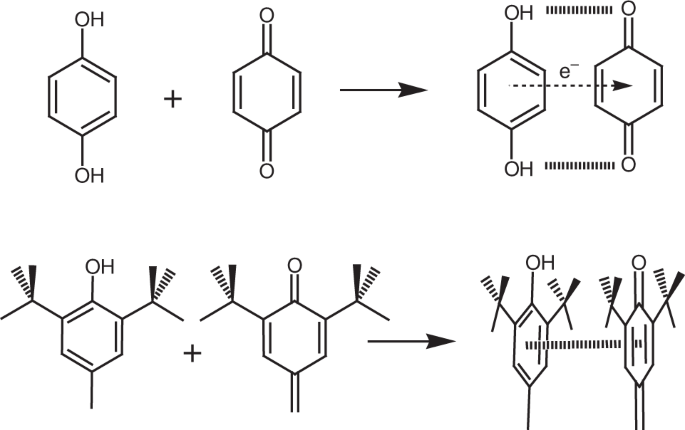
In 2019, we published a paper in Nature Communications describing the synthesis and structures of anti-oxidant-substituted resorcinarenes1. This had been the culmination of a stream of syntheses of other anti-oxidant-substituted chromophores undertaken to attempt access to molecules that possessed oxidation-state-coupled structures similar to that observed for the meso-substituted porphyrin (meso-tetrakis(3,5-di-t-butyl-4-hydroxyphenyl)porphyrin)2. The latter can exist in two different stable oxidation states, essentially analogues of hydroquinone/benzoquinone3, the quinone of which is subject also to tautomeric processes. By the time we had reached resorcinarene, we had realized that certain structural features such as meso-positions situated between macrocyclic aromatic moieties are important to establish oxidation state coupling with structure/conformation. For resorcinarenes substituted with anti-oxidant groups, steric proximity of substituents and the usual conformational selection of resorcinarenes led to the main interest of the compounds. However, our attempts to characterize the compounds also ended in the realization that these compounds might present scope for development based on other properties. That is, the compounds bearing quinone substituents (i.e., higher oxidation states of the compounds) are capable of generating highly reactive singlet oxygen, a species that can be used in several different applications including photodynamic therapy and organic synthesis.
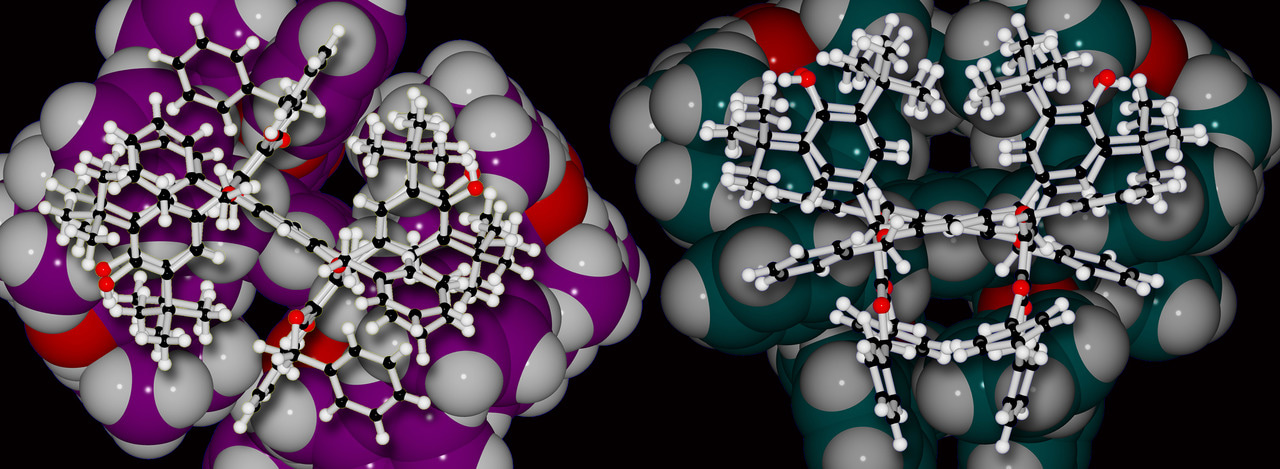
Since the publication of the initial communication of these compounds1, we have been working actively to determine underlying mechanisms of action and reasons why these compounds have this special reactivity under irradiation. Our first work on this subject, in which we described singlet oxygen generating (SO-gen) capabilities of a larger family of anti-oxidant substituted resorcinarenes and pyrogallarenes, was published in 20204. We were extremely lucky in this case to be able to present X-ray crystal structures of almost all the compounds (Figure 1, ‘SO-gen Molecule Zoo’) which provides unequivocal data regarding the structures and has allowed us to establish a new class of compounds, quinone-substituted resorcinarenes, as the Fuchsonarenes4 (by analogy with dyestuff fuchsone5). By the way, Figure 1 also shows members of the SO Team working on quite different aspects of the chemistry – see the caption for a description.
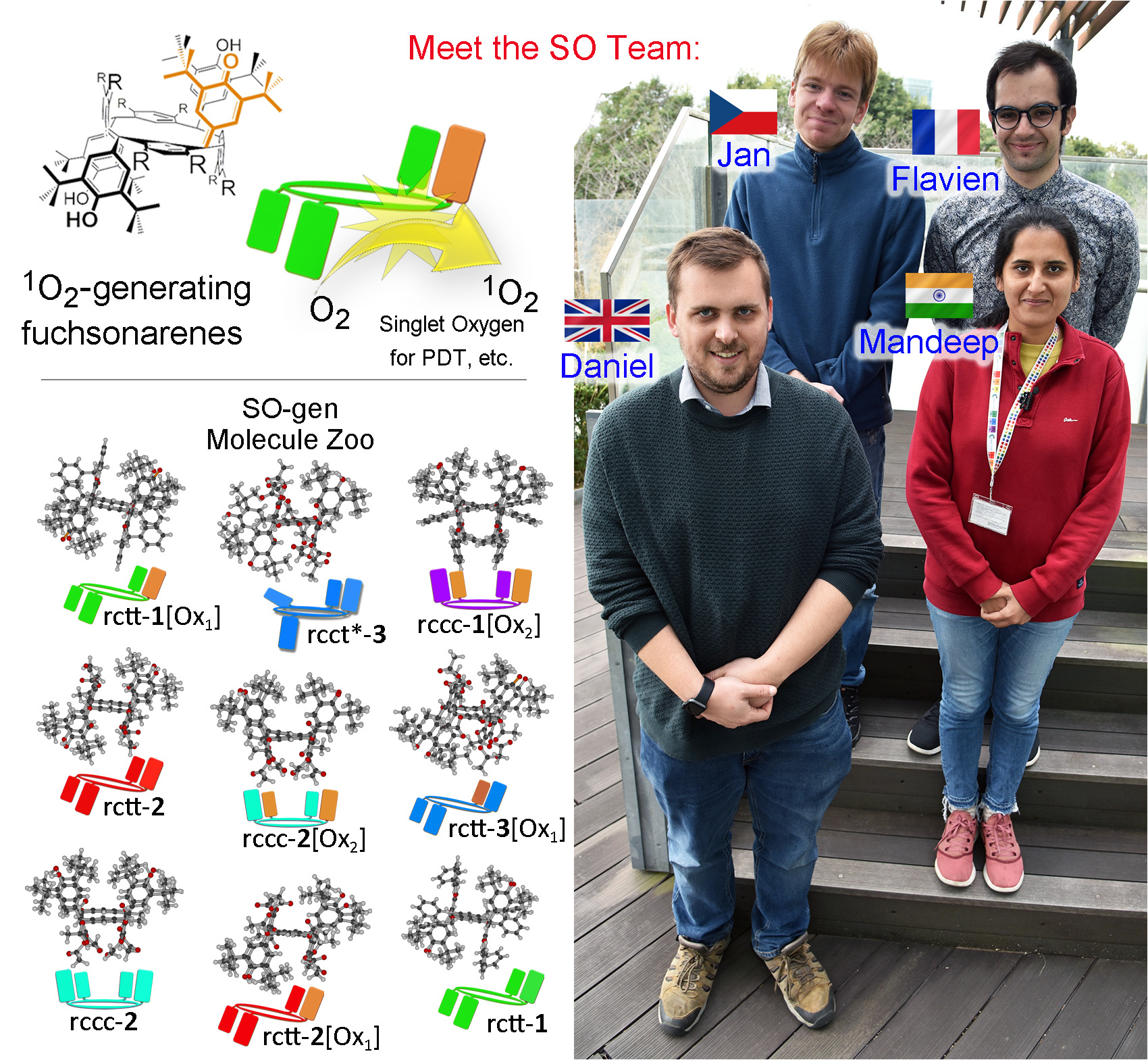
Figure 1. Top left: singlet oxygen generating (SO-gen) fuchsonarenes. Lower left: X-ray crystal structures of a partial Molecule Zoo of the so-far reported compounds. Right: the SO-gen team at NIMS Namiki: anticlockwise from left: Dr. Daniel T. Payne, Dr. Mandeep K. Chahal, Dr. Flavien Sciortino and Dr. Jan Hynek. Daniel, Flavien and Jan came to NIMS through the JSPS Fellowship Scheme, an excellent means to reach Japan as a postdoc. Interested parties contact: Jonathan P. Hill (MANA Project) for further information.
So, basically, we can say that a technical issue with the properties of the compounds from our Nature Communications paper has opened up a rich vein of research for us since we can now aim for concrete applications based on the properties of the Fuchsonarenes. Furthermore, our lesson continues since, having identified the likely active moiety in these compounds, we have been able to extend this research project to several other classes of compound currently under our remit. The post-doc who was the main contributing author to the original paper (Dr. Daniel T. Payne) has now been joined on this project by three further post-doc members who are actively pursuing materials applications of these and other compounds in collaboration with coauthors on the original paper and colleagues at National Institute for Materials Science (NIMS), Tsukuba, Japan. Some of these matters will be reported shortly. Also, because of the success of this work, Daniel has been able not only to extend his stay in Japan at the International Center for Young Scientists (ICYS) but also to secure funds for instrumentation to support this very promising research.
Regarding the scientific aspects of this work, I must point out that years of trial-and-error and some serendipitous timing have led us here. Each compound made provided different insights into the problem at hand, originally to control molecular conformation, a goal that has also partly been accomplished. Finally, having gone through the procedure to make and submit the paper, it is extremely gratifying then to be able to carry on developing the same system even if it is not on the same thread as the original paper. At least we have been able to establish a burgeoning (for us) research theme as a direct result of what we did to publish the original paper.
References
1) Payne D. T., Webre W. A., Matsushita Y., Zhu N., Futera Z., Labuta J., et al. Multimodal switching of a redox-active macrocycle. Nature communications 10, 1007 (2019).
2) Golder A. J., Milgrom L. R., Nolan K. B., Povey D. C. 5,10,15,20-Mesotetrakis(3,5-di-t-butyl-4-quinomethide)porphyrinogen: a highly puckered tetrapyrrolic macrocycle from the facile aerial oxidation of a phenolic porphyrin. Journal of the Chemical Society, Chemical Communications 1751-7153, (1989).
3) Ishihara S., Hill J. P., Shundo A., Ohkubo K., Fukuzumi S., Elsegood M. R. J., et al. Reversible photoredox switching of porphyrin-bridged bis-2,6-di-t-butyl phenols. The Journal of the American Chemical Society 133, 16119–16126 (2011).
4) Payne D. T., Webre W. A., Gobese H., Matsushita Y., Karr P. A., Chahal M. K., et al. Nanomolecular singlet oxygen photosensitizers based on hemiquinonoid-resorcinarenes, the fuchsonarenes. Chemical Science 11, 2614–2620 (2020).
5) Becker H.-D. Preparation of fuchsones. The Journal of Organic Chemistry. 32, 2943–2947 (1967).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Hi,
Great work!!!! Just a quick question on application of oxygen in therapeutics:
how do you control for de-oxygenation in photo-dynamic treatment (PDT)?
On the flip side, people with inborn hemoglobin errors (genetic defects such as sickle cell anemia) for example, can play on technique for corrective measures as well as therapy delivery (my thoughts). This group of people are likely to respond differently to oxygenation for several reasons including malformed red blood cells thus expressing defective signals for oxygen uptake/assimilation. However, with personalized medicine, the oxygen can be conformed in ways that matches the gene defect for efficient uptake and PDT can be applied for diagnostics and therapy delivery.
Lastly, with the highlighted technique in the paper, how to you control for re-dox (reduction and/or oxidation) reactions of the spliced oxygen? Singlet oxygen may not be stable and has the potential of attracting electrons (like/unlike) to its other shell for stabilization thus creating chances of having undesired molecules in your reaction which can play into the composition, precipitation, PH balance and etc of your product. It can also catalyse your reaction making it uncontrollable with chances of a forward/reverse reaction. Is there a need for a control station to work in so as to minimize interference?
Thank you.