Multiple-Stimuli Fluorescent Responsive Metallo-Organic Helicated Cage Arising from Monomer and Excimer Emission
Published in Chemistry

Materials exhibiting luminescence with responsiveness to stimuli have garnered consistent attention in fundamental research and practical applications. These applications span a range of areas, including sensing, bioimaging, data security, and display devices. Following Kasha’s excitonic coupling theory, the photophysical properties of fluorescent materials are directly influenced by both the extent of chromophores aggregation and the relative orientation of chromophores within these aggregates. Metallo-organic cages can be promising platforms for developing stimuli-responsive luminescent materials. However, the precise control of variations for aggregation and dispersion behavior of metallo-orgainc cages induced by external stimulus still presents great chanllenges due to the high complexity of metallo-organic cages in contrast to small conjugated luminescent molecules.
We present a systematically designed axially-twisted metallo-organic cage featuring a triple helicate motif, denoted as MTH [Zn3L2]. This structure was comprehensively characterised through NMR, ESI-MS, and single-crystal X-ray diffraction analysis. In fact, the growth of the single crystal of MTH is not as simple as imagined and spends lots of efforts, despite being a relatively simple structure. MTH displays concentration- and temperature-dependent luminescent behaviour, exhibiting dual emissions of monomer and excimer fluorescence in solution. Its fluorescence emission, tunable from orange to blue, was successfully achieved by adjusting the concentration and temperature. Moreover, single-molecule white light emission was realized in both systems.
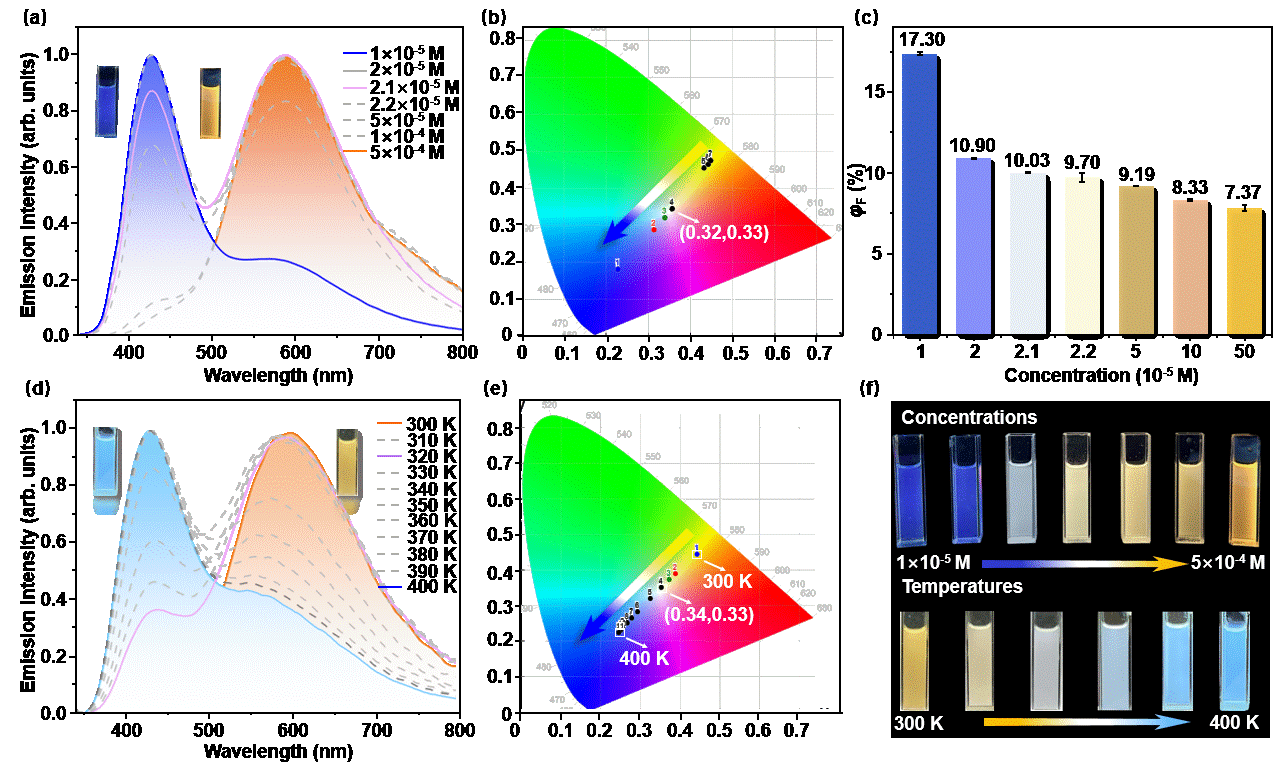
Tunable fluorescence emission of metallo-organic cage MTH at different concentrations and temperatures.
The optical properties of luminescent materials are closely linked to the molecular conformation as well as the stacking mode of inner chromophores. The confirmation from single crystal X-ray diffraction data establishes the distorted conformation of MTH, preventing direct face-to-face π-π staking induced fluoresence quenching. A single axially-twisted pillar in two MTHs adopted a head-to-tail configuration, forming an excimer with moderate stability. This configuration facilitates a switchable supramolecular fluorescence system between monomer and excimer states triggered by varying concentrations or temperatures. A small energy difference between monomer and excimer, experimentally determined to be 6.4 kcal/mol, further demonstrates that MTH excimer can readily transform into monomeric molecules in respond to external stimuli.
Moreover, by incorporating the metallo-organic cage MTH into the thermoplastic materials, specifically PMMA, a dual-mode emission with temperature variation has been successfully realized in the solid state. This achievement has been further applied as a fluorescence temperature indicator and as a thermally-activated information encryption material. It not only broadens the application field of metal-organic supramolecular materials, but also provides ideas for the design and preparation of multifunctional smart luminescent materials.
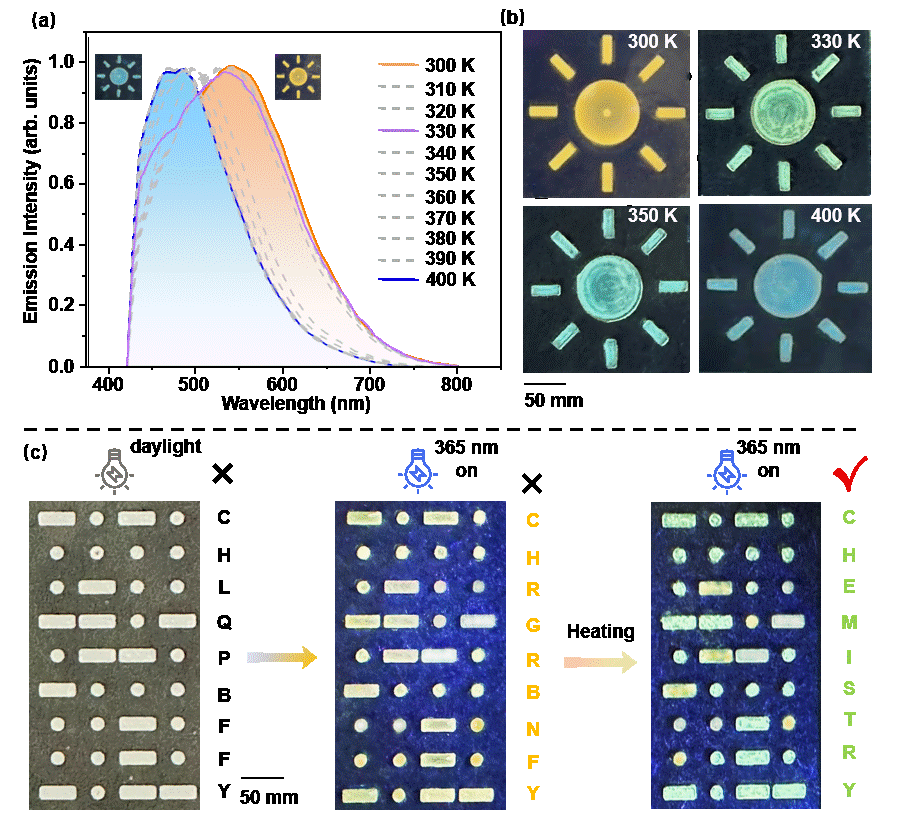
Tunable fluorescence emission in solid state.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in