Explore the Research
N7-methylguanosine methylation of tRNAs regulates survival to stress in cancer - Oncogene
Oncogene - N7-methylguanosine methylation of tRNAs regulates survival to stress in cancer
One of the biggest difficulties in finding an effective cancer treatment is fighting against the ability of tumour cells to survive under a variety of stressful conditions. This capability enables cancer cells to easily adapt to distinct situations and promotes tumour progression and growth, being the main driver of tumour regrowth. As a consequence, the search of efficient molecular targets to overcome these limitations is the major end of thousands of researchers around the globe. In this scenario, RNA modifications or the epitranscriptome arise as a promising target to assess tumour cells survival. Their dynamism and significance in regulating essential cellular processes such as cell proliferation and stress resistance, make the epitranscriptome a compelling prospect to study in the cancer landscape. During the last decades, more than 170 different RNA modifications have been identified in all RNA species, and their relevance in regulating essential cellular mechanisms has been widely proven. Among all RNA species tRNAs are the most heavily modified and these chemical marks have been specifically linked with regulation of tRNA stability and correct molecule folding, being crucial for optimal protein synthesis (1). In fact, dysregulation of tRNA modifications deposition is associated with multiple stress related diseases such as cancer. As in fact, 5-cytosine methylation (m5C) and 7-methylguanosine (m7G) of the variable loop of tRNAs have been described to precisely regulate chemotherapy response of tumour cells (2).
Our work is focused on the study of the role of the methyltransferase METTL1, which is responsible for the m7G deposition at position 46 of certain tRNAs and is overexpressed in a variety of solid tumours, including prostate cancer, as revealed by the group previously. Prostate cancer is characterised by a modest percentage of patients that are refractory to androgen deprivation therapy (ADT) and chemotherapy. Our data revealed higher levels of METTL1 expression in ADT-resistant tumours, suggesting a connection between therapy responsiveness and METTL1 (3). In addition, we successfully identified METTL1 to be mainly expressed in the prostate by luminal, those described to be the preferred cell of origin of prostate cancer. Based on this, in the current Oncogene publication, we further explore this link by investigating how several stressors impact the survival of tumour cells in the presence and absence of the methylase in both in vitro and in vivo conditions. Interestingly, we found that METTL1 depletion decreased the ability of prostate cancer cells to survive under cytotoxic agents, including chemotherapeutic drugs (Docetaxel). These findings confirmed the relationship between RNA modifications and stress responses corroborating previous studies. However, since the molecular mechanism underlying the process has not been clarified yet, the major goal of the current study is to shed some light.
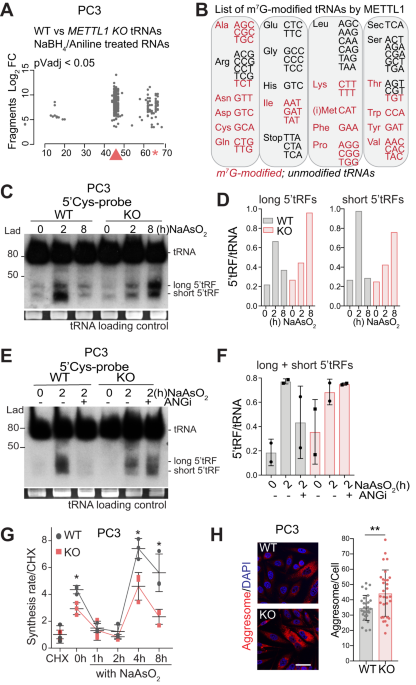
Several studies have highlighted the importance of distinct tRNA modifications in regulating the formation tRNA derived fragments (tRFs). In fact, in our previous work, we successfully detected that loss of METTL1-dependent m7G induced the formation of a specific class of tRFs with an oligo-guanine terminal domain (TOGs) (3). Here, we not only confirmed the fragment formation upon METTL1 deletion but also demonstrated its dependency on stress induction. Interestingly, tRFs accumulation results in a global repression of protein synthesis as well as alterations of the translational program. Detailed analysis of the altered stress response pathways revealed a dysregulation of autophagy termination, resulting in an accumulation of autophagosomal particles and protein aggregates in the cell cytoplasm. In addition METTL1-depleted cells also exhibited dysregulation of the redox balance, which led tothe accumulation of intracellular reactive oxidative species (ROS), as well as dysregulation of DNA repair mechanisms which resulted in DNA damage accumulation that leads to increased apoptosis and cellular senescence. As a consequence of these pathways’ dysregulations and increased intracellular stress, the ability of cancer cells to effectively respond to stressors, including chemotherapy treatments, diminishes, as demonstrated,in both in vitro and preclinical prostate cancer models. Interestingly, we also found that by targeting its METTL1 methyltransferase activity, we could also reduce tumour cell aggressiveness and proliferation, as well increased their sensitivity to common chemotherapy agents such as Docetaxel and Etoposide.
In summary, our findings underscore the crucial role of m7G tRNA methylation in shaping cancer stress responses. Furthermore, we shed light on the underlying molecular mechanism driven by the accumulation of tRFs, which impairs a cell's ability to adapt to stress and consequently heightens its sensitivity to therapy. Consequently, METTL1 emerges as a promising therapeutic target with the potential to enhance the efficacy of conventional anti-tumour drugs used in clinical settings, particularly for patients who have proven resistant to standard treatments. Notably, the recent development of a METTL3 inhibitor, a member of the METTL family, suggests that the development of a METTL1 inhibitor is not only feasible but also highly plausible, although further research is warranted to explore this avenue.
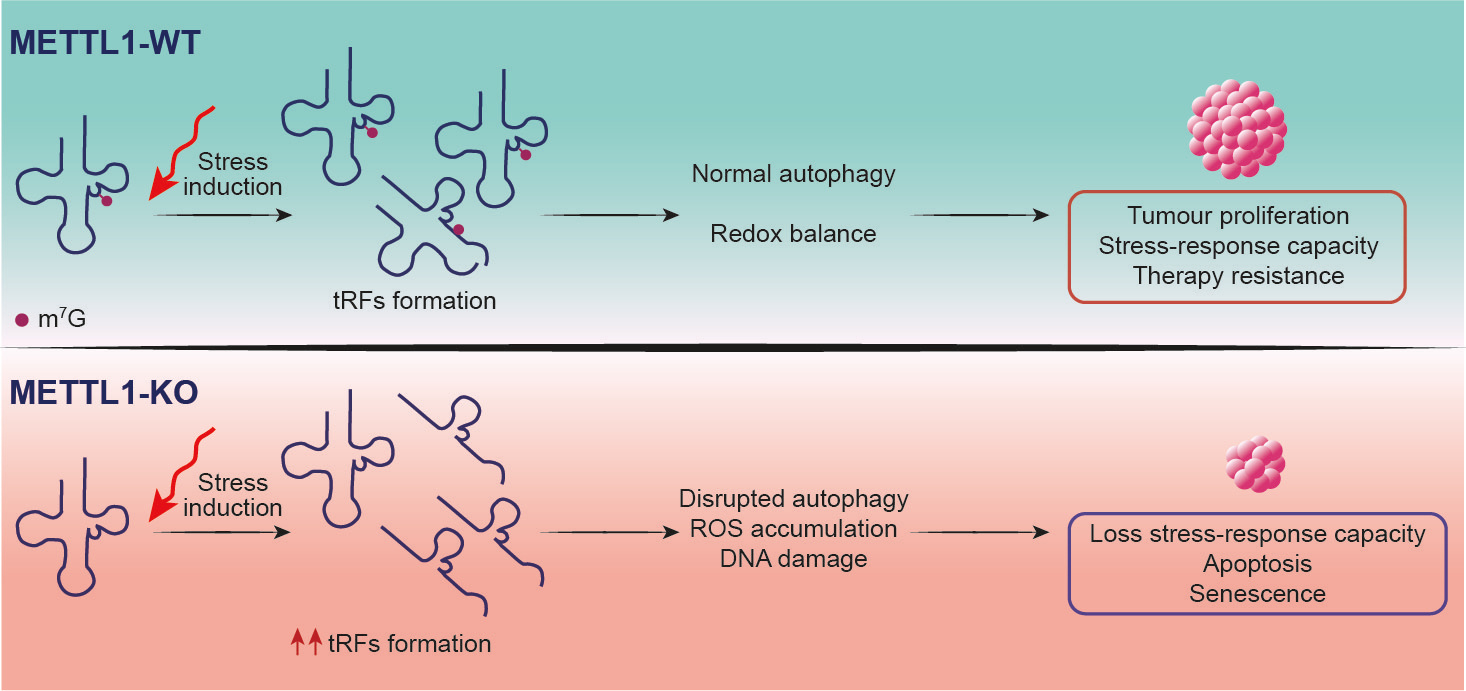
Figure 1. Schematic representation of the effect of METTL1 deletion in cancer cells under stress conditions. In the absence of the methyltransferases, the tRNAs target of METTL1 lack the m7G mark and, under stress conditions, are subjected to selective cleavage and formation of stress-induced tRNA-derived fragments (tRFs). This condition results in a alteration of the translation program and alteration of stress response pathways such as autophagy. As a consequence,there is an alteration of the redox balance that results in ROS accumulation, which, in turn, lead to an increase of the DNA damage resulting in an elevated intracellular stress. In this scenario, tumour cells loss their ability to respond to extracellular stresses and are more prone to apoptotic and senescence programs, that ultimately leads to increased sensitivity to chemotherapy agents and reduced tumour proliferation.
References
- Delaunay S, Frye M. RNA modifications regulating cell fate in cancer. Nat Cell Biol. 2019 May;21(5):552-559.
- Okamoto M, Fujiwara M, Hori M, Okada K, Yazama F, Konishi H et al. tRNA modifying enzymes, NSUN2 and METTL1, determine sensitivity to 5-fluorouracil in HeLa cells. PLoS Genet 2014; 10: e1004639
- García-Vílchez R, Añazco-Guenkova AM, Dietmann S, López J, Morón-Calvente V, D'Ambrosi S et al. METTL1 promotes tumorigenesis through tRNA-derived fragment biogenesis in prostate cancer. Mol Cancer. 2023 Jul 29;22(1):119.
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in