Navigating the Complexity of Multicomponent Heteroleptic Assembly: A Journey into Thermodynamic Self-Sorting of Coordination Cages
Published in Chemistry
Introduction
The self-assembly of supramolecular coordination cages, mimicking biological systems such as receptors and enzymes, has captivated the attention of synthetic chemists. In a recent Nature Chemistry paper, our team delves into the progressive assembly of multicomponent [Pd2ABCD] cages, composed of two palladium ions and four different organic bridges, inspired by the sophisticated and low-symmetry nano-confinements found in folded biopolymers. This work stands at the crossroads of coordination chemistry and supramolecular self-assembly, pushing the boundaries of what can be achieved in creating precisely shaped, non-statistically assembled multicomponent structures.
Inspiration from Nature
Drawing inspiration from the complex shapes and functionality seen in enzymes, which utilize folded peptide chains to create selective recognition sites and catalytic centres around nanoscopic cavities, our team sought to replicate these concepts in the form of synthetic molecular assemblies based on metal nodes and organic bridging ligands. Building on the foundational work of Lehn, Sauvage, Fujita, and others1–5, recent advancements in metallosupramolecular assembly provided the tools for achieving the clean and exclusive formation of multi-component low-symmetry structures by the combination of multiple building blocks in a non-statistical fashion. Previously, a number of integrative self-sorting strategies, such as shape-complementary assembly, coordination sphere engineering, selective backbone interactions and the use of multidentate donor environments opened new avenues for achieving a stepwise increase of structural complexity and functionality in the synthetic realization of discrete nano-objects. We now showed that a combination of several of these strategies is able to lift this approach to the next level6.
Challenges in Achieving Multicomponent Assemblies
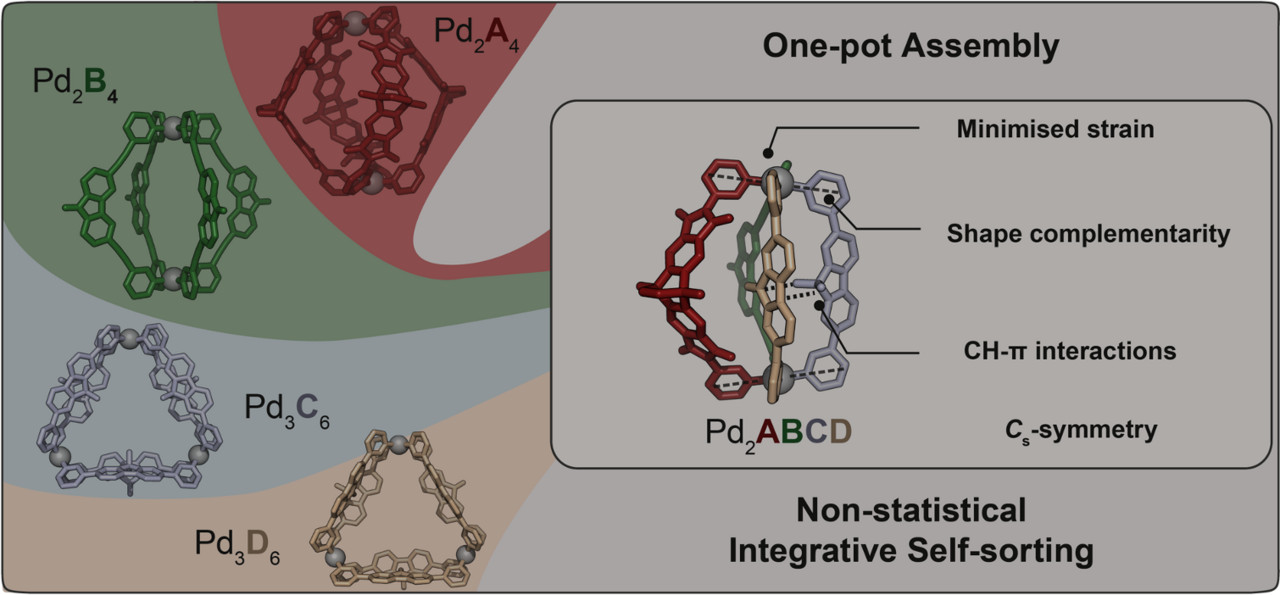
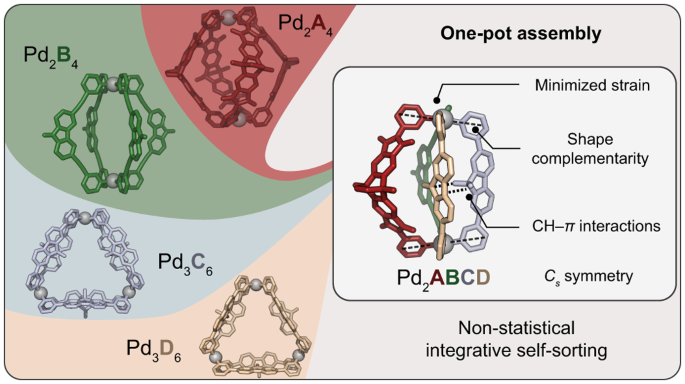
The non-statistical synthesis of heteroleptic (= composed of several different ligands) coordination cages is challenging, particularly from a thermodynamic perspective. Imagine attempting to assemble a dinuclear structure from four distinguishable bis-monodentate bridging ligands, A, B, C, and D, each capable of independently coordinating to two square-planar PdII nodes. Theoretically, without careful control, this system could result in 55 different discrete species, from the homoleptic (= all ligands the same) assemblies over numerous binary and ternary combinations up to three isomeric structures containing all four different ligands.
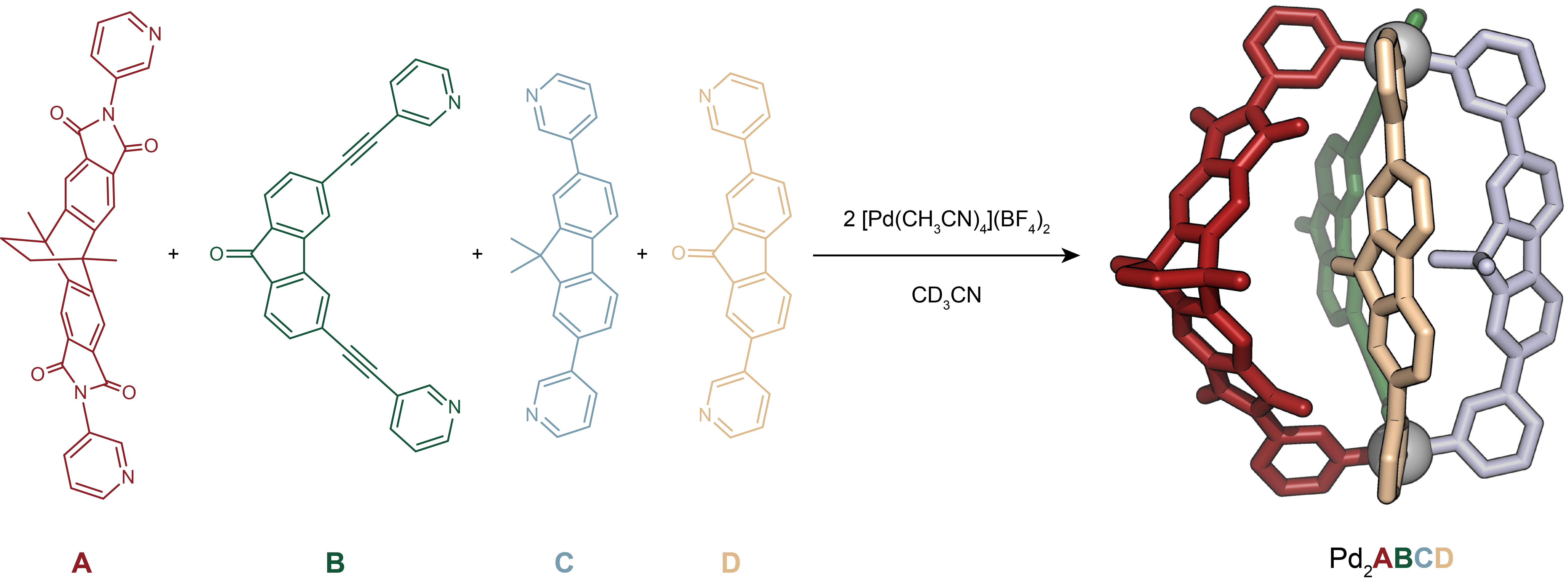
Figure 1. Self-assembly of heteroleptic multicomponent cage [Pd2ABCD]4+ from four chemically different ligands. Cage formation from ligands A, B, C, D and PdII and X-ray structure of [Pd2ABCD]4+.
A Diverse Array of Heteroleptic Cages
Our systematic approach resulted in a family of heteroleptic cages, where two square-planar PdII cations are bridged by various combinations of ligands A, B, C, and D. Heteroleptic cages with two, three, and eventually four different ligands were successfully assembled, demonstrating the potential for selective and controlled assembly. The most challenging assembly involved the simultaneous incorporation of four chemically different ligands, however, all based on simple pyridyl donors, resulting in the exclusive formation of compound [Pd2ABCD]4+ (Figure 1). The X-ray structural analysis as well as experimental and computational thermodynamic studies revealed intriguing details about the orientation and interactions between the ligands.
Understanding Selectivity and Self-Sorting
Concerning the rationale behind the selectivity of ligand interactions, our study highlighted the importance of C-H⋯π interactions and shape-complementarity between the individual ligands (Figure 2). Control experiments, crystal structures, and computational analyses provided insights into the preferences and dynamics of the ligands in the assembly process. The study also explored the generality of forming heteroleptic cages with various ligand variations.
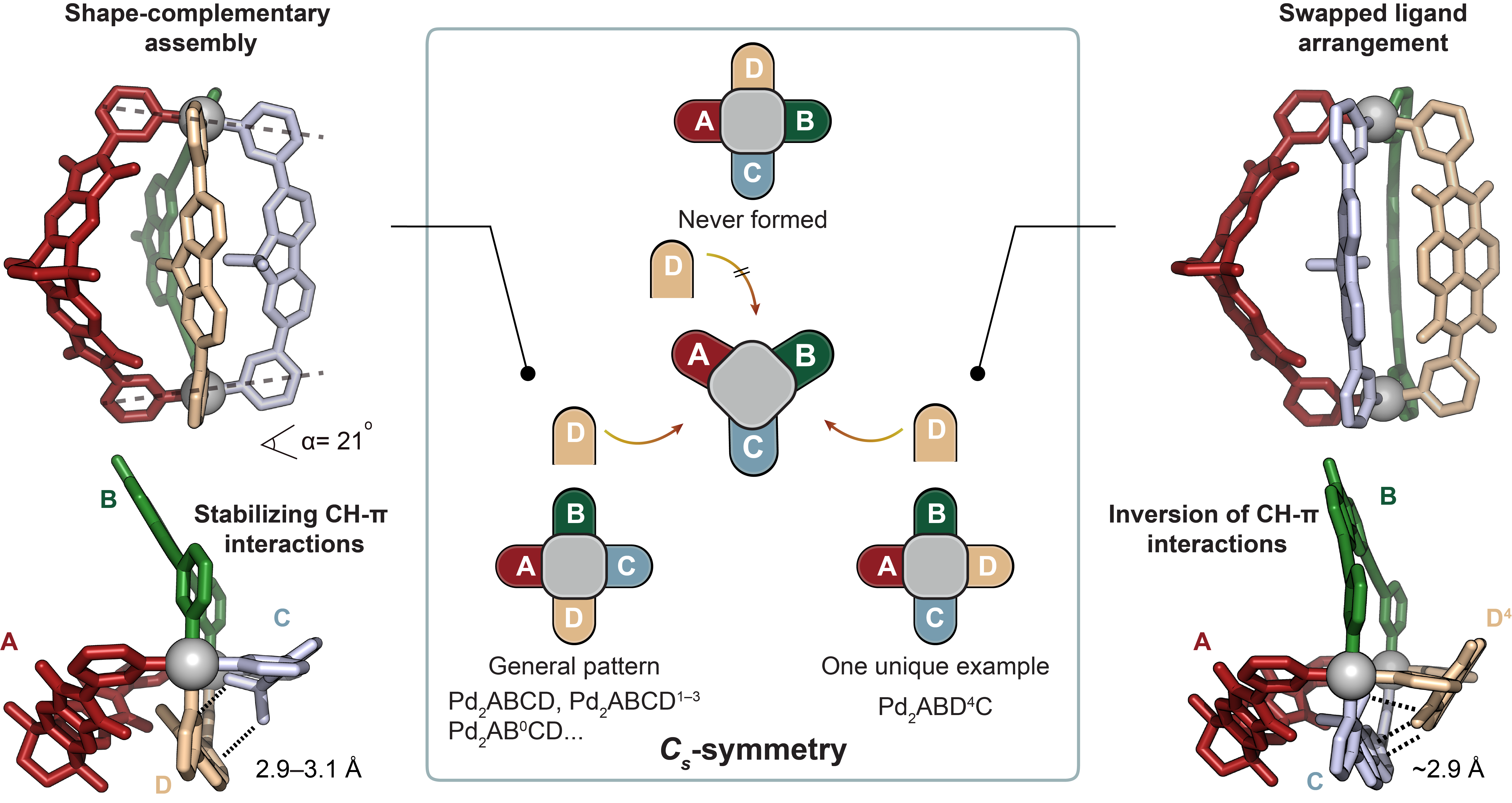
Figure 2. Possible cage isomers and comparison of crystal structures of [Pd2ABCD] and [Pd2ABD4C]. Left: side and top X-ray views of cage [Pd2ABCD]4+ (dihedral angle α between [Pd(Py)4] planes shown to indicate the presence of pronounced shape-complementarity and distances of C-H⋯π interactions between C and D assigned). All the ligand combinations with D, and D1–D3 led to the general ABCD pattern. Middle: Three configurational isomers with Cs-symmetry arranging four different ligands circularly around two metal nodes shown. Right: side and top X-ray views of [Pd2ABD4C] showing a swapped ligand arrangementand inversion of C-H⋯π interactions (C-H⋯π distance between ligands D4 and C given).
Progressive assembly of Multicomponent Heteroleptic Cages
The mixing of cage solutions with lower complexity levels led to the clean transformation into two-component, three-component, finally four-component cages. Notably, we demonstrated that the formation of the most complex cage, [Pd2ABCD]4+, could be achieved either through stepwise procedures or directly by mixing all four homoleptic assemblies (Figure 3). Van’t Hoff analyses revealed that each step in the transformations, from level II to III to IV, is energetically downhill, highlighting the thermodynamic control exerted, the robustness and reproducibility over the self-assembly process. Every step is more complex yet every step afford more stable assemblies. This showcases a significant advancement in designing and understanding the thermodynamic aspects of complex multicomponent supramolecular assemblies.
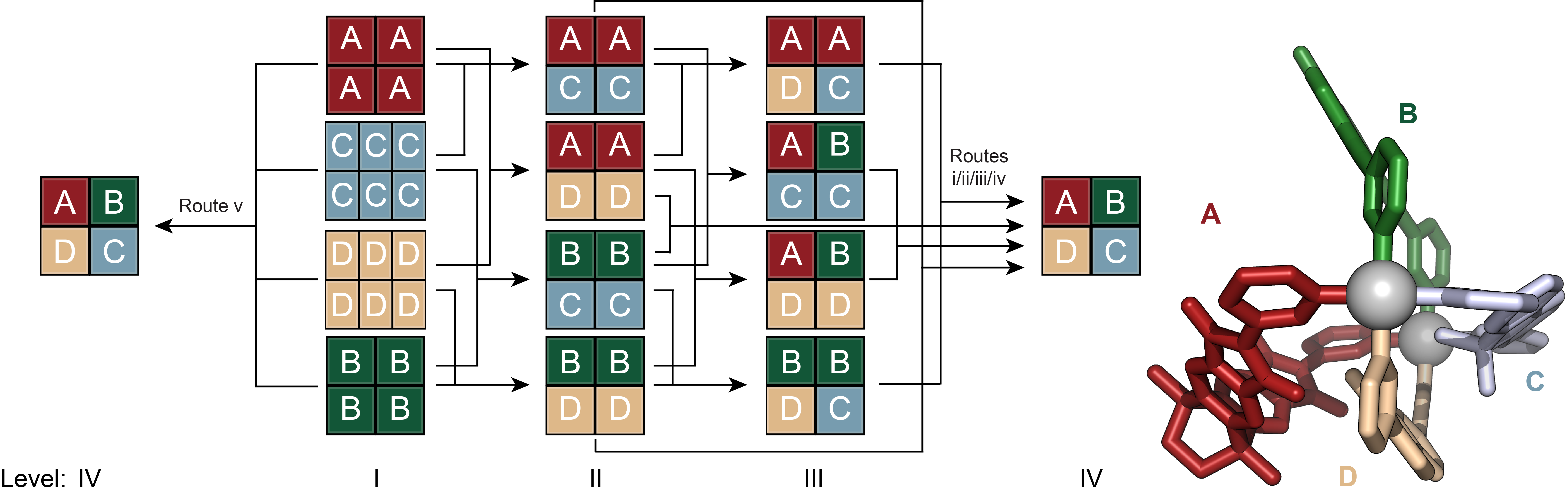
Figure 3. Self-assembly of heteroleptic multicomponent cage [Pd2ABCD]4+ from four chemically different ligands. Evolution of complexity from homoleptic assemblies to the most complex heteroleptic assembly [Pd2ABCD]4+ via different pathways.
Conclusion and Outlook: A Sophisticated Control and Complexity
While a large deal of reported metallosupramolecular assemblies exhibit high symmetry derived from Platonic or Archimedean solids, our work addresses the rational synthesis of low-symmetry heteroleptic cages with the highest complexity ever. In contrast to homoleptic assemblies, where a single type of ligand dominates, our study focuses on the controlled integration of up to four different ligands without creating statistical mixtures. This level of control and complexity is achieved through a combination of shape-complementarity, inter-ligand interactions, and strain. Beyond structural considerations, the study acknowledges the challenges and potential in incorporating functionality into these assemblies. Drawing inspiration from biological systems where enzymes utilize the precise spatial positioning of chemical functionalities for recognition and catalysis, our work opens the way to explore the potential of these heteroleptic cages for applications in selective recognition, cooperative catalysis, and materials science, mirroring the adaptability and specificity seen in biomolecules.
Future challenges include expanding the scope of ligand variations, introducing functionalities such as fluorescence, chirality, redox etc, understanding the dynamics of cage-to-cage transformations, and exploring the emergent properties that arise from the interplay of different functionalities within these multifunctional assemblies.
- Krämer, R., Lehn, J. M. & Marquis-Rigault, A. Self-recognition in helicate self-assembly: spontaneous formation of helical metal complexes from mixtures of ligands and metal ions. Proc. Natl. Acad. Sci. USA 90, 5394–5398 (1993).
- Sauvage, J. P. & Weiss, J. Synthesis of biscopper(I) [3]-catenates: multiring interlocked coordinating systems. J. Am. Chem. Soc. 107, 6108–6110 (1985).
- Kumazawa, K., Biradha, K., Kusukawa, T., Okano, T. & Fujita, M. Multicomponent Assembly of a Pyrazine-Pillared Coordination Cage That Selectively Binds Planar Guests by Intercalation. Angew. Chem. Int. Ed. 42, 3909–3913 (2003).
- Zheng, Y.-R. et al. A Facile Approach toward Multicomponent Supramolecular Structures: Selective Self-Assembly via Charge Separation. J. Am. Chem. Soc. 132, 16873–16882 (2010).
- Wessjohann, L. A., Kreye, O. & Rivera, D. G. One-Pot Assembly of Amino Acid Bridged Hybrid Macromulticyclic Cages through Multiple Multicomponent Macrocyclizations. Angew. Chem. Int. Ed. 56, 3501–3505 (2017).
- Pullen, S., Tessarolo, J. & Clever, G. H. Increasing structural and functional complexity in self-assembled coordination cages. Chem. Sci. 12, 7269–7293 (2021).
Website:
Google Scholar:
https://scholar.google.com/citations?user=r1EhFRcAAAAJ&hl=en
Orcid:
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in