NCATS Tissue Chips in Space-The UW Kidney Chip Experiences
Published in Social Sciences and General & Internal Medicine
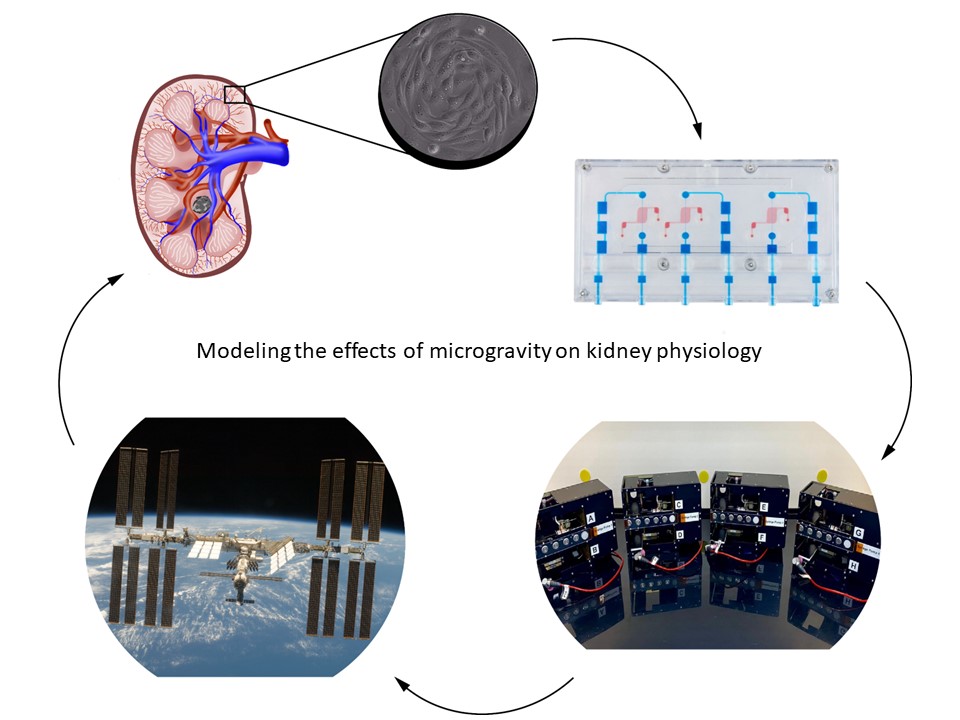
The National Center for Advancing Translational Sciences (NCATS) was established in 2011; the first funding initiative was the Microphysiological Systems (MPS) Program in 2012. The goal of this program was to create “tissue chips” or avatars of human organs as an alternative to experimentation on preclinical animal species to better predict drug safety and toxicity. As part of this initiative, our team at the University of Washington and the Kidney Research Institute created an MPS model of the human kidney. In 2016, NCATS partnered with Center for the Advancement of Science in Space (CASIS) for the “Tissue Chips in Space” program to send MPS models to the International Space Station National Laboratory (ISS-NL). The rationale for this initiative was based on the observation that microgravity elicits accelerated aging effects on the human body; by using this unique environment we can better understand human diseases to improve health on Earth. Our group was selected in 2017 to send a kidney MPS to the ISS-NL to study kidney physiology and the role of the kidney in osteoporosis and kidney stone disease. In this “Behind the Papers” post we describe the challenges faced to modify the kidney MPS perfusion system from a terrestrial lab to the ISS-NL for our two successful launches in 2019 (CRS-17) and 2022 (CRS-22) as well as the experimental results from CRS-17.
Our group at the University of Washington and the Kidney Research Institute was part of the NCATS/CASIS partnership for Tissue Chips in Space (link: Tissue Chips in Space | National Center for Advancing Translational Sciences (nih.gov)). We were selected in 2017 for two launches to the ISS-NL to study the effect(s) of microgravity on human kidney physiology using our MPS model of the kidney proximal tubule. In the first launch we studied the responses to proteinuria (the presence of excess serum proteins in urine) and metabolic activation of the precursor form of vitamin D. The second launch modeled nephrolithiasis (kidney stone disease) by exposure of MPS to calcium oxalate microcrystals, the principal mineral component of kidney stones. The rationale for modeling proteinuria is based on the increased risk of glomerular dysfunction in microgravity and subsequent excessive serum proteins in the filtrate. In the case of vitamin D, the kidneys are responsible for generating biologically active vitamin D (1α,25(OH)2D3) which has pleiotropic activities including maintaining bone mineralization/density which is known to be adversely affected in microgravity. The decreases in bone mineralization and density in microgravity place astronauts at increased risk of developing kidney stones by elevating serum calcium concentrations, so we assessed the early molecular and cellular effects of calcium oxalate microcrystal exposure.
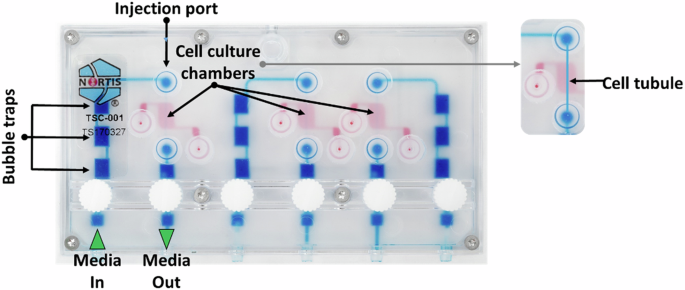
The first challenge we faced in these studies was generation of a miniaturized hardware perfusion system compatible with the volume restrictions of cell culture facilities available on the ISS-NL that was not gravity-dependent. In the first of our two publications in npj Microgravity Development of a kidney microphysiological system hardware platform for microgravity studies - PubMed (nih.gov), we describe the development of the kidney chip perfusion platform (KCPP). With implementation partner, BioServe Space Technologies BioServe Space Technologies | University of Colorado Boulder, we created the KCPP in 18 months that reduced the footprint required to support 24 triple channel MPS 8-fold. In addition, the KCPP incorporated syringe pump perfusion, as the MPS perfusion system used terrestrially is driven by a pneumatic system incompatible with microgravity. Because crew time in the ISS-NL is extremely limited, the controls for the KCPP were simplified to include only necessary operations; the final KCPP has only five buttons that can control all the functions required to maintain the devices and conduct experiments. In its final form, the KCPP is a modular system that can hold up to twelve MPS devices in a single locker-sized SABL unit in the ISSNL. The KCPP provides automated fluid delivery, temperature control, and gas exchange. During both CRS-17 and CRS-22, the KCPP successfully maintained our MPS devices in ground-based laboratories, during transit, and on station. While we consider both of our launches successful, we faced significant mold contamination of MPS on CRS-17. This was likely due to a number of factors including repeated launch delays requiring multiple media changes in the lab at Kennedy Space Center as well as a scrub of our launch on May 3rd, 2019. The launch scrub required us to offload our experiment from the Dragon capsule, return to the lab and reload on May 4th, 2019, when CRS-17 launched at 2:48 AM EDT. Upon return to Earth and de-integration at our lab in Seattle, WA we observed ~25% of the MPS had mold contamination, affecting both flight and ground samples. In response, we developed new mold mitigation control protocols for CRS-22 and faced no delays in our launch on June 3rd, 2021 at 1:29 PM EDT; no MPS were found to have mold contamination upon de-integration after CRS-22. For both flights, MPS were allowed to acclimate to microgravity at the ISS-NL for ~1 week followed by 2 days exposure to the various test articles and then subsequent preservation at -80°C before return to Earth and shipping to our lab.
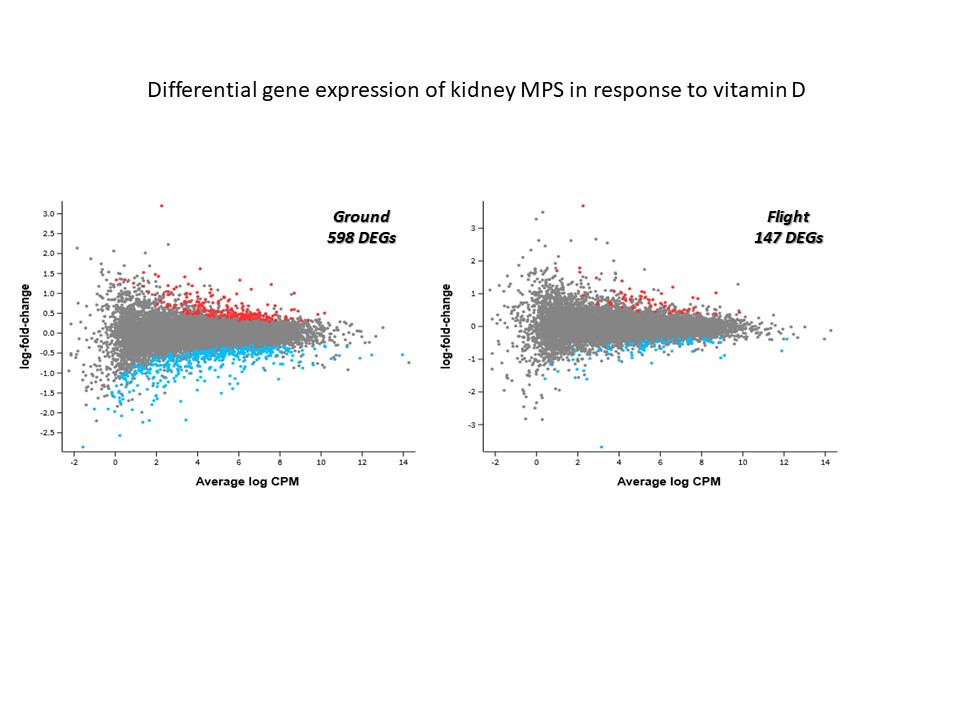
In our second publication in npj Microgravity Modeling cellular responses to serum and vitamin D in microgravity using a human kidney microphysiological system - PubMed (nih.gov) we describe the results from the experiments conducted by Astronauts Christina Koch and Anne McCain on CRS-17. We launched 24 MPS (with equal ground controls) comprised of 72 independently perfused tubules seeded with kidney cells from four different donors (two of each sex). Despite loss of some samples to mold contamination, we were able to generate and analyze useful data. The data was comprised of changes in gene expression using bulk RNAseq as well as effluent biomarkers. We have previously shown that MPS effluents contain the same protein biomarkers observed in the urine of patients with acute kidney injury (kidney injury molecule-1 (KIM-1) or the inflammatory cytokine interleukin-6 (Il-6)). Following exposure to 2% normal human serum as a model of proteinuria we observed ~2200 differentially expressed genes (DEGs) for both flight and ground MPS. When considering perturbed pathways or disease processes, we saw results which replicated previous work published by our group but there were no unique responses separating flight from ground environments following serum exposure. These results suggest that microgravity does not modulate the kidney response to proteinuria, at least over a short term (48 hour) exposure. As expected, KIM-1 and Il-6 effluent biomarkers were significantly elevated after serum exposure but there were no statistically significant differences between flight and ground. Treatment of MPS with the precursor form of vitamin D (25(OH)D3) bound to its cognate carrier vitamin D binding protein produced similar results to serum exposure - no unique genes or pathways were identified when comparing flight and ground. In addition, both flight and ground MPS produced comparable levels of the active form of vitamin D as well as other expected metabolites. KIM-1 and Il-6 were both elevated, albeit to lower levels compared to serum (which was expected). Of note, we observed statistically significant lower levels of Il-6 protein induction in flight MPS versus ground (which was consistent with mRNA levels) following 48 hours of treatment with 25(OH)D3 but the reason(s) behind this are unknown. Finally, when comparing the number of DEGs following treatment with 25(OH)D3 it is interesting that there were ~4 times fewer DEGs in flight versus ground (147 and 598, respectively). Previous studies have shown that astronauts exhibit lower circulating levels of the active form of vitamin D in microgravity despite 25(OH)D3 supplementation; our results may corroborate this with the caveat that the duration of time in microgravity in our experiment (~1 week) before treatment with 25(OH)D3 is a fraction of a typical 6-month ISS-NL mission. We are currently studying viability and function of our MPS for at least 6 months with NASA support (Extended Culture of Kidney MPS and Organoids to Model Acute and Chronic Exposure to Drugs and Environnmental Toxins - NASA Science). With the success of these ground-based studies, we hope to conduct similar duration studies in microgravity to better predict the long-term effects on astronaut health and develop mitigation strategies as we look to the future of crewed lunar and Mars missions.

Follow the Topic
-
npj Microgravity

This journal aims to provide a thorough understanding of the scientific impact and future of spaceflight research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Human System Risk Management and Knowledge Graphs for Human Spaceflight - Volume II
Publishing Model: Open Access
Deadline: Oct 31, 2026
Space Biomanufacturing
Publishing Model: Open Access
Deadline: Jun 15, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in