New insights into malaria vaccine immune mechanisms - opening the door to achieving higher efficacy?
Published in Microbiology, General & Internal Medicine, and Immunology

Explore the Research
Just a moment...
www.thelancet.com needs to review the security of your connection before proceeding.
Malaria burden, vaccine limitations
Malaria is a leading cause of childhood morbidity and mortality, and negatively impacts education, equity, and economic development in endemic countries. Concerningly, the global estimates of malaria cases and deaths have not declined since 2015, when the WHO malaria elimination goals were renewed, and disease burden is now higher than in 2019. Combating malaria is a target of the UN Sustainable Development Goals (SDGs).
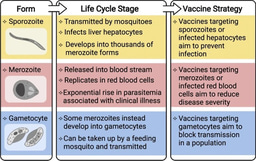
In October, 2021, WHO recommended the first malaria vaccine for children (first dose at age 5–6 months) in areas with moderate-to-high Plasmodium falciparum transmission. This vaccine, known as RTS,S, is predicted to have significant public health benefits, especially when used in combination with insecticide-treated nets and antimalarial drugs. However, vaccination only confers 55-70% efficacy over 12 months and wanes within 18 months. In malaria endemic populations, it has only been shown to be consistently efficacious in young children and not older children or adults. New insights are needed to enable the development of vaccines with higher efficacy and longevity and vaccines that can be used among all age groups. (see Figure 1 below).
Understanding how the RTS,S vaccine works, and why it is not more efficacious and long-lasting may reveal strategies to achieve the goal of more effective vaccines. However, the immunological mechanisms of RTS,S-mediated immunity are poorly understood. Therefore, we aimed to identify antibody response types associated with protection against malaria in children vaccinated with RTS,S.
To address this knowledge gap we investigated immune mechanisms among children aged 1–4 years who were vaccinated with RTS,S in a phase 2b clinical trial conducted in Mozambique.
An overview of the RTS,S vaccine can be found here
New insights on potential protective mechanisms of antibodies
Antibody specificity and type strongly influence the ability of antibodies to form immune complexes and mediate functional activities via the antibody fragment crystallisable (Fc) region (see Figure 2 below). This includes the ability of IgM and IgG to fix serum complement component C1q, which activates the classical complement pathway and can enhance antibody-mediated inhibition of sporozoite motility or invasion and lead to sporozoite lysis. IgG can also bind Fcγ receptors (FcγRs) to promote opsonic phagocytosis of sporozoites, which is primarily mediated by neutrophils and to a lesser extent monocytes, and activate natural killer cells. RTS,S induces IgM and IgG, especially the IgG1 and IgG3 subclasses, which are cytophilic and can strongly fix complement and bind FcγRs.
The RTS,S vaccine encodes a part of the P. falciparum circumsporozoite protein (CSP), which is the major surface antigen of the sporozoite stage. Sporozoites are inoculated by a feeding mosquito and travel through the skin and circulation to the liver where they can establish infection. The aim of the RTS,S vaccine is to target sporozoites and prevent infection. Sporozoites might be susceptible to antibody recognition and Fc-dependent functional activities in the skin after inoculation and in the blood before reaching the liver.
Our analyses of immune responses in young African children found that greater IgA responses to two regions of the CSP protein (known as the NANP-repeat and C-terminal regions) were associated with higher vaccine protection. Additionally, antibodies to the CT region that had higher Fc-mediated functional activities were also associated with a significantly reduced risk of malaria. These functions include antibodies that that fix complement, and bind to FcγRIIa and FcγRIII. Children with multi-functional antibodies to the CT region had a reduced risk of malaria, while higher multifunctional antibodies to the NANP repeat region were not associated with better vaccine protection.

Associations between RTS,S-induced antibodies responses and time to first clinical malaria episode. Sera from children in the RTS,S vaccine group collected 30 days after vaccination were tested for various antibody responses to antigens representing the NANP (blue) and CT (grey) regions of CSP. Analyses were performed for all participants (A), males (B), and females (C), adjusting for age. aHR=adjusted hazard ratio. CSP=circumsporozoite protein. CT=C-terminal. Multi=multifunctional antibodies. NANP=Asn-Ala-Asn-Pro repeats. OD=optical density. ∗p<0·05. †p<0·01.
IgA responses – how could they protect against malaria?
Our finding that IgA responses were associated with greater vaccine efficacy was unexpected and intriguing. IgA is the second most abundant immunoglobulin isotype in serum (after IgG), but it is often overlooked in malaria vaccine studies.
We found a significant induction of IgA to the NANP-repeat and C-terminal regions of CSP. Notably, higher IgA magnitudes were significantly associated with a reduced risk of malaria, which has not been reported in children before. Providing further evidence of the potential protective role of IgA, we show that RTS,S-induced antibodies bind the Fcα-receptor, which is expressed on certain phagocytic cells, and can mediate opsonic phagocytosis. In our initial experiments, we demonstrated phagocytosis by neutrophils; these are the most abundant phagocytes in blood and our previous studies demonstrated that these cells play an important role in phagocytosis IgG-opsonised sporozoites. Future studies are needed to explore other cells that express FcαRI, such as monocytes and macrophages. Anti-CSP IgA has been shown to inhibit sporozoite invasion in vitro, which might be an additional mechanism by which RTS,S-induced IgA could contribute to protection.
Further research is needed to understand the potential role of IgA in immunity. Mice are the primary animal model used for preclinical evaluation of malaria vaccines; however, they lack FcαR, have low complement activity, and have different FcγR functions and expression. This highlights critical differences between mouse and human immunity relevant to understanding vaccine efficacy in children presenting challenges in comparing findings between human and mouse studies.
Are there sex-based differences in mechanisms of vaccine protection?
Sex is increasingly recognised as an important factor that influences vaccine responses and many differences in immune functions between males and females have been described.
To investigate the potential impact of sex on RTS,S immunity, we stratified our analyses by sex. This revealed different associations between antibody responses and protection against malaria in male and female participants. We found notable differences in the associations between antibody responses and protection against malaria in male and female participants using univariate and multiparameter machine-learning analytical methods. For example, Protective associations were stronger for male participants than female participants for functional antibody responses to the C-terminal region. Only IgA responses were significantly associated with protection among female participants.
Intriguingly, our findings suggest that the mechanisms of protection might differ among males and females and could explain why it has been difficult to define a strong correlate of protection as vaccine trial findings are rarely, if ever, stratified by sex. The mechanisms explaining these differences need to be defined but might include differences in the expression and functions of Fc receptors among boys and girls or functions of immune cells such as neutrophils and monocytes, which have been poorly studied in malaria-endemic populations.
Conclusions and future
Our recent studies provide new evidence that functional antibody responses mediated by IgG and IgA are associated with protection against malaria in young children vaccinated with RTS,S, and suggest potential differences in the correlates of immunity between males and females. These findings reveal new avenues that could be used to achieve malaria vaccines with higher efficacy. Our findings are also relevant for the R21 vaccine, which is based on the same antigenic construct as RTS,S and has recently been recommended for implementation in young children. Our findings encourage research on potential approaches that could be used to enhance IgA responses and Fc-mediated protective functions of IgG. Additional larger studies in other populations are also needed to build knowledge and inform future strategies.
Full article can be found here:
https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(24)00130-7/fulltext
Detailed review of the RTS,S vaccine can be found here:
https://www.science.org/doi/10.1126/scitranslmed.abo6646
Figures

Figure 1: Overview of the RTS,S vaccine. From Beeson JG et al, Science Translational Medicine, 2022

Figure 2: When evaluating malaria immunity, antibody responses can be categorized into 1) standard antibody parameters, 2) antibody Fc-dependent functions, and 3) antibody inhibitory functions. From Opi DH et al, Expert Review of Vaccines, 2021 - https://www.tandfonline.com/doi/full/10.1080/14760584.2021.1981864
Related articles :
https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-022-02466-2
https://www.nature.com/articles/s41467-021-21998-4
https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-019-1277-x
https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-018-1054-2




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in