New research unveiling the invisible puppeteers: how our gut microbiota pulls the strings on host locomotion.
Published in Microbiology, Neuroscience, and Anatomy & Physiology
Have you ever considered that the tiny bacteria in your gut might be puppeteers controlling your movements? Imagine there is tempting food placed before you; it would be hard to control the impulse to grab it when you are hungry. Hunger drives us to approach food, but how does the gut transmit signals of hunger and satiety to the brain and thus influence behavior? Our latest study reveals a mechanism by which the gut commensal bacteria mediate the glucagon-like peptide-1 (GLP-1), a gut hormone known for regulating appetite, and consequently leads to altered host movement.

Hunger drives us to approach food, but how does the gut transmit hunger and satiety signals to the brain and thus change the behavior?
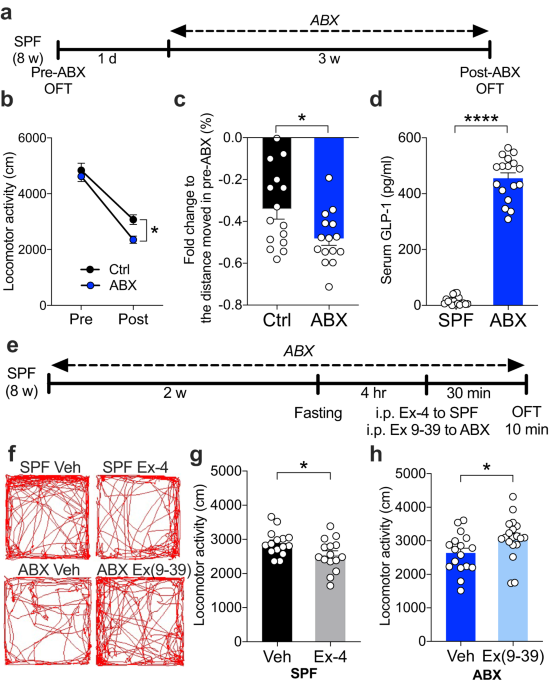
In our study, when we administered the broad-spectrum antibiotic cocktail to effectively deplete the gut microbiota in mice, it was intriguing that the mice decreased their movement distance compared to the group with complete gut microbiota. These microbiota-depleted mice had higher levels of the “satiety gut hormone” GLP-1 in their blood. Prompted by these findings, we explored the connection between GLP-1 and movement. We administered a GLP-1 receptor agonist to the mice with complete gut microbiota and observed a decrease in their locomotion. Conversely, inhibiting GLP-1 signaling in microbiota-deprived mice reversed their reduced movement. It is becoming clear: GLP-1 plays a key role in controlling movement.
One crucial issue is the connection between the gut and the brain. The vagus nerve, serving as a critical communication pathway between the gastrointestinal tract and the brain, appears to be a key player. We examined the neuron activity in response to this hormonal elevation after the behavior test and found the area postrema (AP) in the brainstem with increased activity in the antibiotic-treated mice. AP is also a region where the vagus nerve, a major component of the parasympathetic nervous system, relays signals to the brain. With GLP-1 receptors present on the vagal terminals, the vagus nerve could be a crucial mediator in this gut-brain communication pathway. To test this hypothesis, we applied two distinct methods. One involved surgically resecting the vagus nerve, which restored normal movement in the antibiotic-treated mice. The other applied focused ultrasound as a non-invasive approach to stimulate the dorsal vagal complex area in the brainstem region in the mice with a complete gut microbiota, which also resulted in reduced locomotion. A clear picture emerged: the vagus nerve significantly orchestrates this gut-brain dialogue, similar to the strings between the puppet and its controller.
Another objective we try to explore is to pinpoint specific bacteria influencing GLP-1 levels. After observing the GLP-1 levels in response to different selective antibiotics, only two types of antibiotics led to significantly increased GLP-1. The sequencing of 16S ribosomal RNA in the fecal samples from selective antibiotic-treated mice revealed the alterations in bacterial composition between different antibiotic groups. We identified bacterial species, Lactobacillus reuteri and Bacteroides thetaiotaomicron, which are correlated with GLP-1 levels in the selective antibiotic-treated groups. Finally, we colonized the microbiota-depleted mice with the two bacterium candidates. Remarkably, we observed a reduction of GLP-1 levels and a restoration of locomotor activity. It appears that we identify the puppeteers controlling the host locomotor behavior. Our study demonstrates that the specific microorganism orchestrates locomotor activity via the GLP-1 signaling and the vagal-dependent pathway.
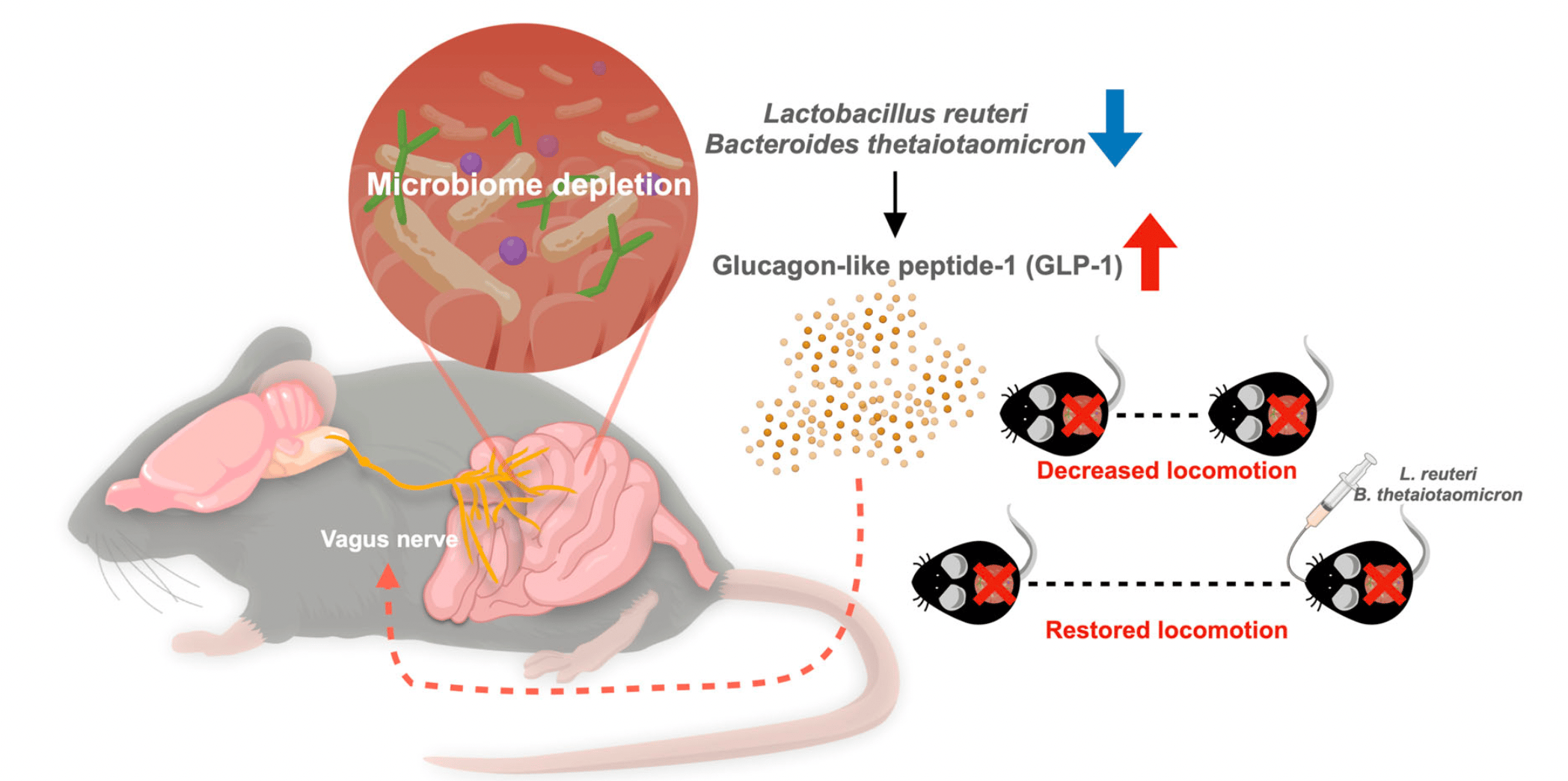
The concept of how microbial-mediated GLP-1 modulates host movement.
This study all began with a coincidence. We discovered that antibiotic-treated mice had a subtle decrease of movement and wondered why. What followed was an unexpected and multifaceted journey for discovery. We performed rigorous behavioral tests, mastered the intricacies of vagotomy surgery, and delved deep into microbiome sequencing. Collaborations were forged in the pursuit of understanding, including the transcranial focused ultrasound technique and the germ-free mice bacterial colonization. The unexpected COVID-19 pandemic made everything even more challenging, particularly with the animal facility facing the possibility of emergency shutdowns. Fortunately, we have finally completed this study and pleased to share it with you.
Our gut bacteria are active participants in host physiology; they have the potential to influence host actions with substantial effects. Research on the gut-brain axis has flourished in recent decades. However, multi-dimensional experiments must be conducted to prove or exclude potential hypotheses and pathways when investigating such a complex issue. Of course, the development of new technology is an advantage we can apply in designing our strategies, such as the focused ultrasound stimulation used in this study to specifically activate the brain region in a non-invasive way Combining all the techniques allows us to see the big picture on a more micro scale.
Curious about the complete details of our research? Check out our full paper for an in-depth exploration, “The gut microbiota modulate locomotion via vagus-dependent glucagon-like peptide-1 signaling”, published in npj Biofilms and Microbiomes. Discover more at: https://doi.org/10.1038/s41522-024-00477-w.
(Poster image was created with generated AI.)
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: May 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in