Not all sulfonyl fluorides were created equally - some have oxetanes
Published in Chemistry
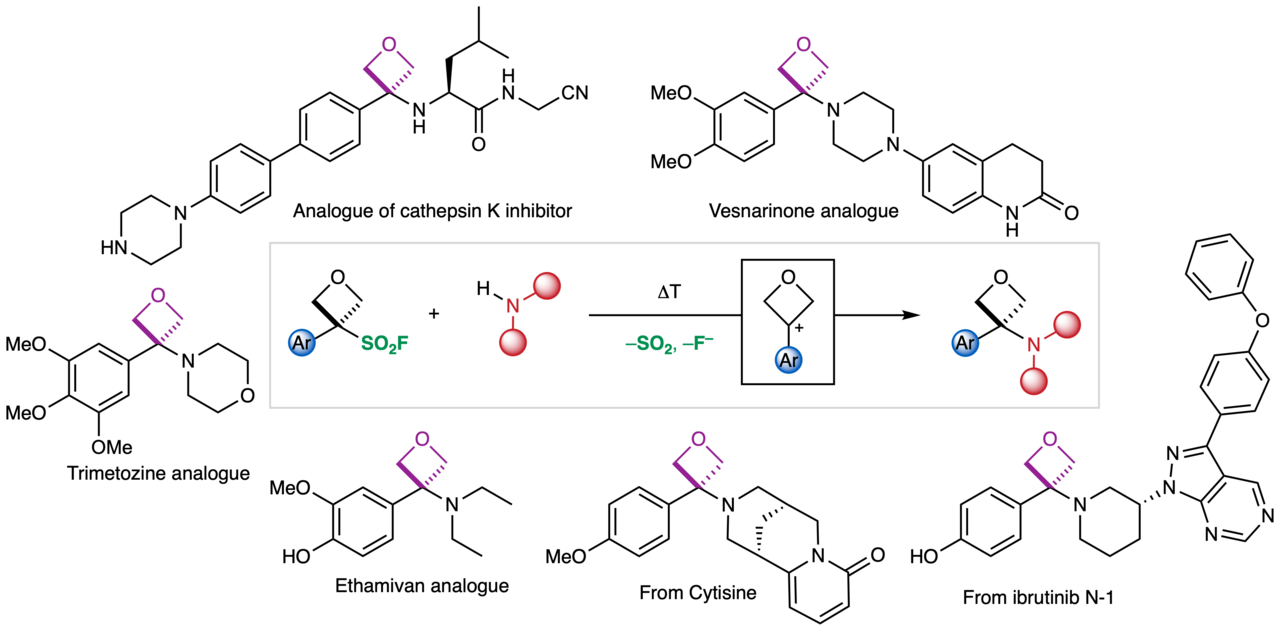
In the Bull Group we have a long-standing interest in developing synthetic methods to access under-represented small polar motifs valuable to drug discovery, particularly 4-membered rings. An ongoing objective has been to establish an efficient route to aminooxetanes, which could be direct isosteres of amides, amides being the most common functional group in medicinal chemistry. We were interested in a reaction to directly couple an oxetane precursor to amines to mimic the most used reaction in medicinal chemistry, the amidation reaction. We found a solution to this in an unlikely place!
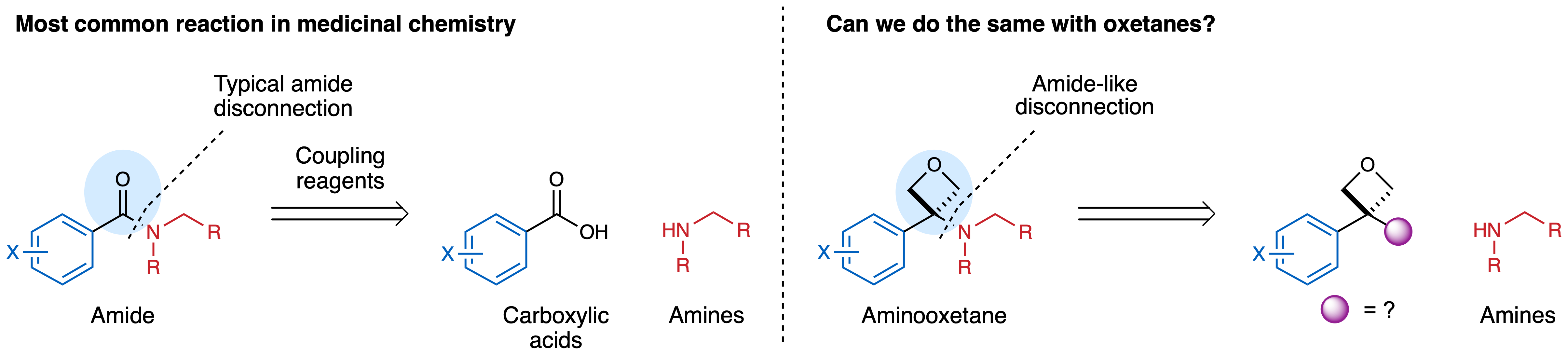
In our previous work, as part of this ongoing collaboration with Pfizer, we functionalised oxetanol derivatives by hydroxyl activation with Lewis acids to enable reaction with phenols and thiols. However, with amine nucleophiles, the amine is too Lewis basic and prevented any reaction. At the same time we were targeting reactive sulfonyl derivatives to prepare new oxetane building blocks. Rosie Croft made the first oxetane sulfonyl fluoride back in 2017. Notably, attempts to form oxetane sulfonyl chlorides were unsuccessful (which was perhaps telling!), whereas the oxetane sulfonyl fluorides were much more stable. We envisaged this reagent would react with amines in a sulfur fluoride exchange (SuFEx) reaction to generate oxetane sulfonamides. To our surprise, it did not give the sulfonamide. Instead, under slightly elevated temperatures, the oxetane sulfonyl fluoride lost SO2 and a fluoride ion to form an oxetane carbocation that was trapped by the amine nucleophile to generate an aminooxetane. Careful optimisation indicated that the aminooxetane was best formed under mild basic conditions.

This led to an extensive study on the preparation of oxetane amines, which we report today in Nature Chemistry. The defluorosulfonylation is promoted simply by warming and the sulfonyl fluoride provides a stable and readily activated carbocation precursor. With our coworkers we showcased its broad applicability with over 80 examples of aminooxetanes, including 10 analogues of benzamide drugs, the late-stage functionalisation of complex amines and array chemistry.
We wondered why of all sulfonyl fluoride substituents, the aryloxetanes behaved differently. The SuFEx reaction was discovered long ago in Germany in the 1920’s with a notable 82-page article by Wilhelm Steinkopf: “Über Aromatische Sulfofluoride”. However, sulfonyl fluorides were truly resurrected by Nobel Laureate Barry Sharpless when, in 2014, he demonstrated their potential in “Another Good Reaction for Click Chemistry”. Since then, Sharpless and others have disclosed a plethora of applications of SuFEx chemistry in synthesis, as covalent warheads and affinity labels in chemical biology, and in polymer chemistry. These applications rely on the central characteristic of sulfonyl fluorides, that the S–F bond is very stable (“nearly moribund” as Sharpless would say) but can be “sparked to life” (another Sharpless idiom) with the right assistance.
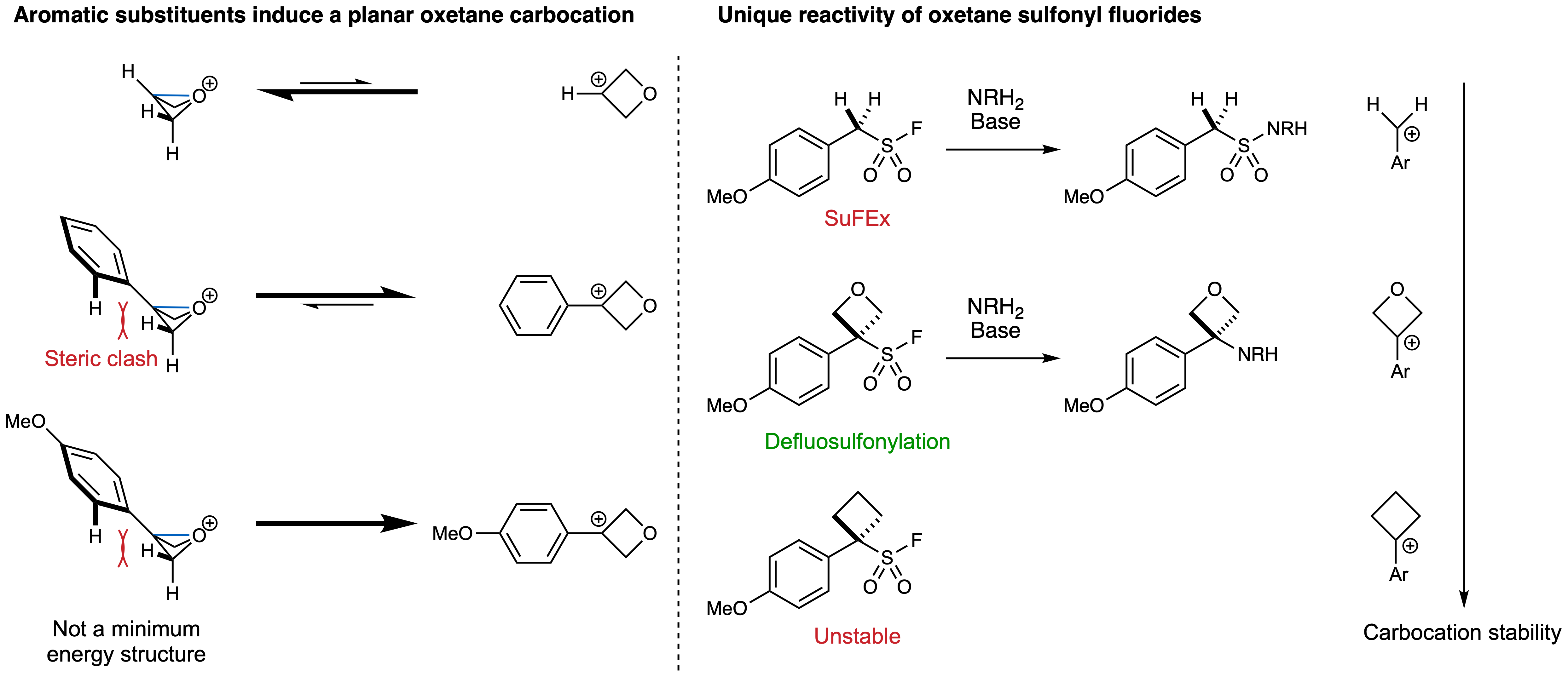
This behaviour all changes with oxetane sulfonyl fluorides which are not in the least bit interested in doing SuFEx chemistry and we wanted to understand why. To do this, we followed the reaction by in situ NMR using varying amounts of each component to work out the reaction orders (using variable time normalisation analysis (VTNA) developed by Jordi Burés). The reactions were prepared in an NMR tube in our lab on the 5th floor and quickly brought down to Peter Haycock in the lower basement, who inserted the sample into the preheated probe, shimmed, locked and started collecting spectra in record-breaking time. With this information we elucidated that the reaction was first order in oxetane sulfonyl fluoride and zero order in everything else, supporting an SN1 mechanism that forms an oxetane carbocation upon loss of SO2 and F–. Indeed, the carbocation was the main ion detected by mass spectrometry of the oxetane sulfonyl fluorides.

These results sparked the next exciting collaboration with computational physical organic chemists Alistair Sterling and Fernanda Duarte at the University of Oxford. Our shared interest was two-fold: 1) What is the nature of the proposed strained oxetane carbocation? Is it bicyclic, invoking stabilisation from the oxygen lone pairs and reminiscent of the strain-release chemistry popularised by Phil Baran and others? Or is it planar, to maximise resonance stabilisation? And 2) What makes oxetanes special to provoke the unique behaviour we observe? Together we responded to both questions. 1) The carbocation of unsubstituted oxetane clearly prefers a bicyclic structure but this preference is reversed with the 3-phenyl-oxetane carbocation which now favours a planar conformation. This is because the planar conformation in the latter improves resonance stabilization and minimizes steric clashes that would otherwise exist between the ortho C–H bonds and the methylene groups on oxetane. This preference is only amplified with para-methoxyphenyl where the bicyclic conformation is not even a minimum energy structure. 2) When comparing the relative stabilities of different benzylic carbocations and at the same time the reactivity of the corresponding sulfonyl fluorides, it became evident that these oxetanes inhabit a privileged reactivity/stability region: the carbocation is stable enough to be formed (unlike those obtained from primary benzylic sulfonyl fluorides) but is not so stable that it is formed spontaneously at room temperature (4-methoxyphenylcyclobutane sulfonyl fluoride is unstable at ambient temperature). We think this defluorosulfonylative pathway may serve as a general explanation for the anecdotal instability of alkyl sulfonyl fluorides.
The SuFEx pathway is additionally hindered by the steric bulk around the sulfur. This becomes evident with phenyloxetane sulfonyl fluoride which is solid as a rock and refuses to react with amines. We saw this all change with phenolates which were able to convince oxetane sulfonyl fluorides to SuFEx. In fact, we can choose between SuFEx with phenols or defluorosulfonylation with amines within the same molecule by simply changing the base.
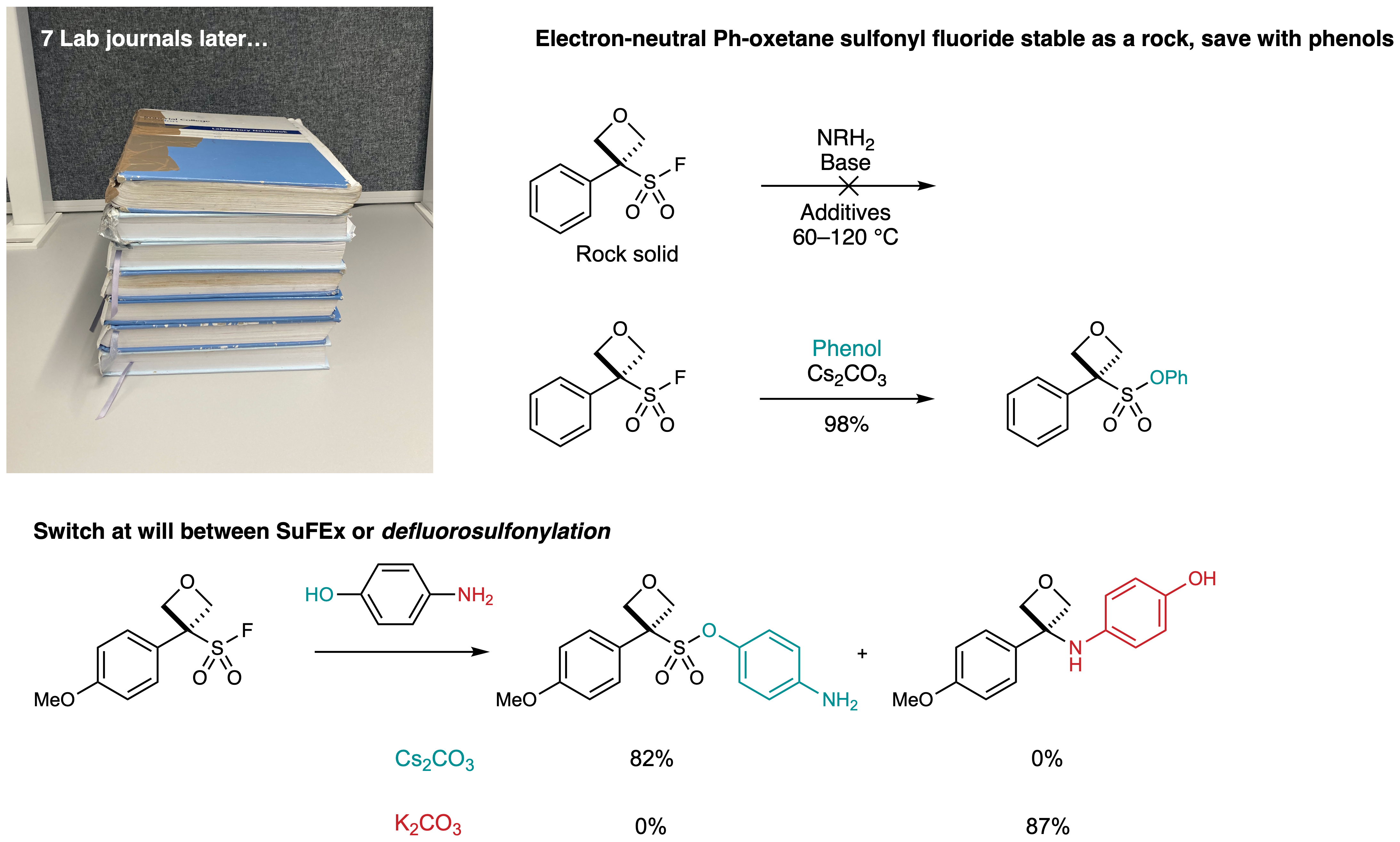
7 lab journals, 4 years, 3 institutions and 13 researchers later we are happy to understand the reactivity of oxetane sulfonyl fluorides rather better!
For the full report please see: https://www.nature.com/articles/s41557-021-00856-2
For more from the Bull group: https://twitter.com/jamesabull
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in