On the origin of life: Endocytosis-like DNA uptake by cell wall-deficient bacteria
Published in Microbiology

A stunning 2.5 to 4 billion years ago, in a period known as the Archaean Eon, the first forms of life appeared on Earth. These primordial organisms were likely simple cells enclosed in a cell membrane, in contrast to the diverse and complex microbes we know today. It is thought that horizontal gene transfer (HGT), or the exchange of genetic material between cells, took place on a large scale1. One way for HGT is the uptake of free DNA from outside of the cell. Present-day bacteria need sophisticated machinery that consists of many proteins to bind to and ‘import’ external DNA across their protective cell wall and cell membrane. How could the early forms of life take up DNA or other particles, when specialized uptake machineries had not yet evolved?
Studying L-forms to learn about ancient cells
As it is difficult to study microbes that no longer exist, scientists use model systems to get as close to these lifeforms as possible. One of those model systems are cell-wall deficient bacteria called L-forms. What are L-forms? Most bacteria possess a cell membrane that encloses their cytoplasm and are surrounded by a protective and shape-defining cell wall. However, during salt stress or in the presence of cell-wall targeting antibiotics, many bacteria can transiently lose their cell wall, allowing them to replicate in this wall-free state.
What can L-forms teach us about uptake processes of early lifeforms? What we have discovered, and is published this week in Nature Communications, is that the transition into an L-form state enables cells to naturally take up external DNA! Even more surprisingly, the L-form cells take up DNA without using proteins with homology to a well-studied and conserved DNA-import system. Cells without a cell wall, and apparently without this uptake system, could still take up DNA.
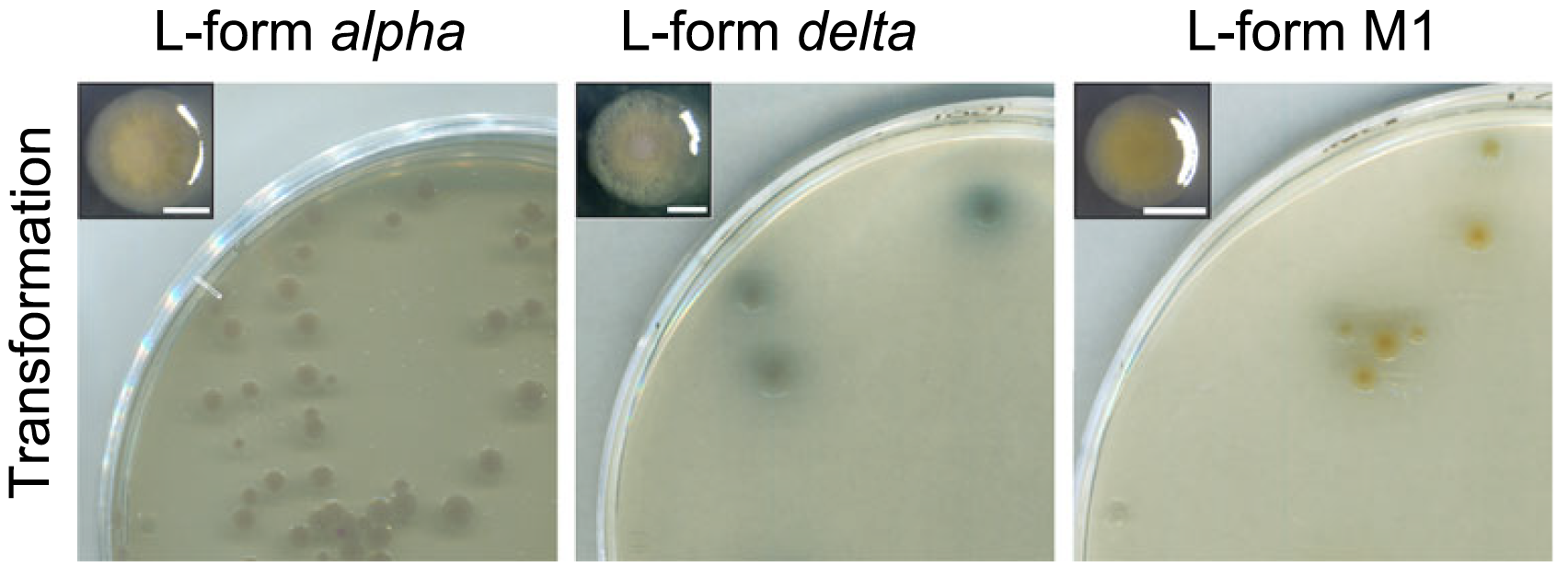
We went on to identify the mechanism by which L-forms take up DNA. Earlier studies showed that eukaryotic cells can take up extracellular materials, including DNA, by a process called endocytosis2. It works by engulfing extracellular fluids, particles or even entire bacteria by the inward bulging of the membrane. A vesicle is formed, and its contents are degraded, for example to release compounds in the cell for food or to get rid of harmful bacteria3,4. Vesicles also can fuse with the cell membrane, for example to recycle receptor molecules.
Interesting! But what does this have to do with the L-forms?
Well, if you look closely at L-forms using a microscope, we could see round vesicles inside these cells. In L-forms that were producing GFP in the cytoplasm (GFP, or green fluorescent protein, emits green light upon excitation with a specific light wavelength – similar to black light at a party that causes your white clothes to shine), these vesicles were dark. As if there was no cytoplasm present in these intracellular vesicles. Could these perhaps be endocytosis-like vesicles containing extracellular liquid?
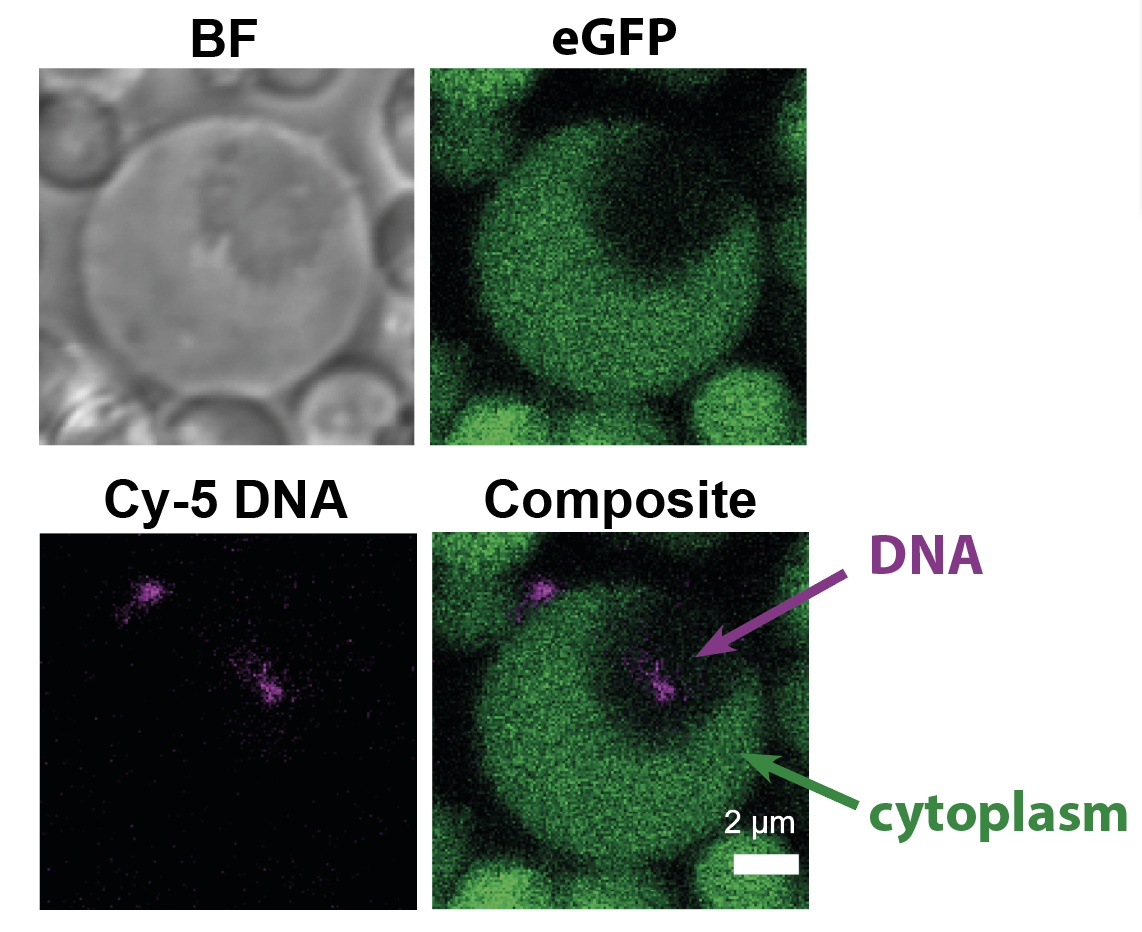
L-form strain expressing eGFP in its cytoplasm, containing Cy5-labeled DNA (magenta) in an internal vesicle (black region; no eGFP).
To test this, we incubated L-forms with all sorts of particles that were tagged with fluorescent markers – so we could trace them inside the cell. Interestingly, fluorescently-labelled DNA ended up inside the vesicle structures! We found that these vesicles were indeed formed by the inward movement of the cell membrane, enclosing extracellular liquid present outside of the cell, and in this way ‘swallowing up’ external material.
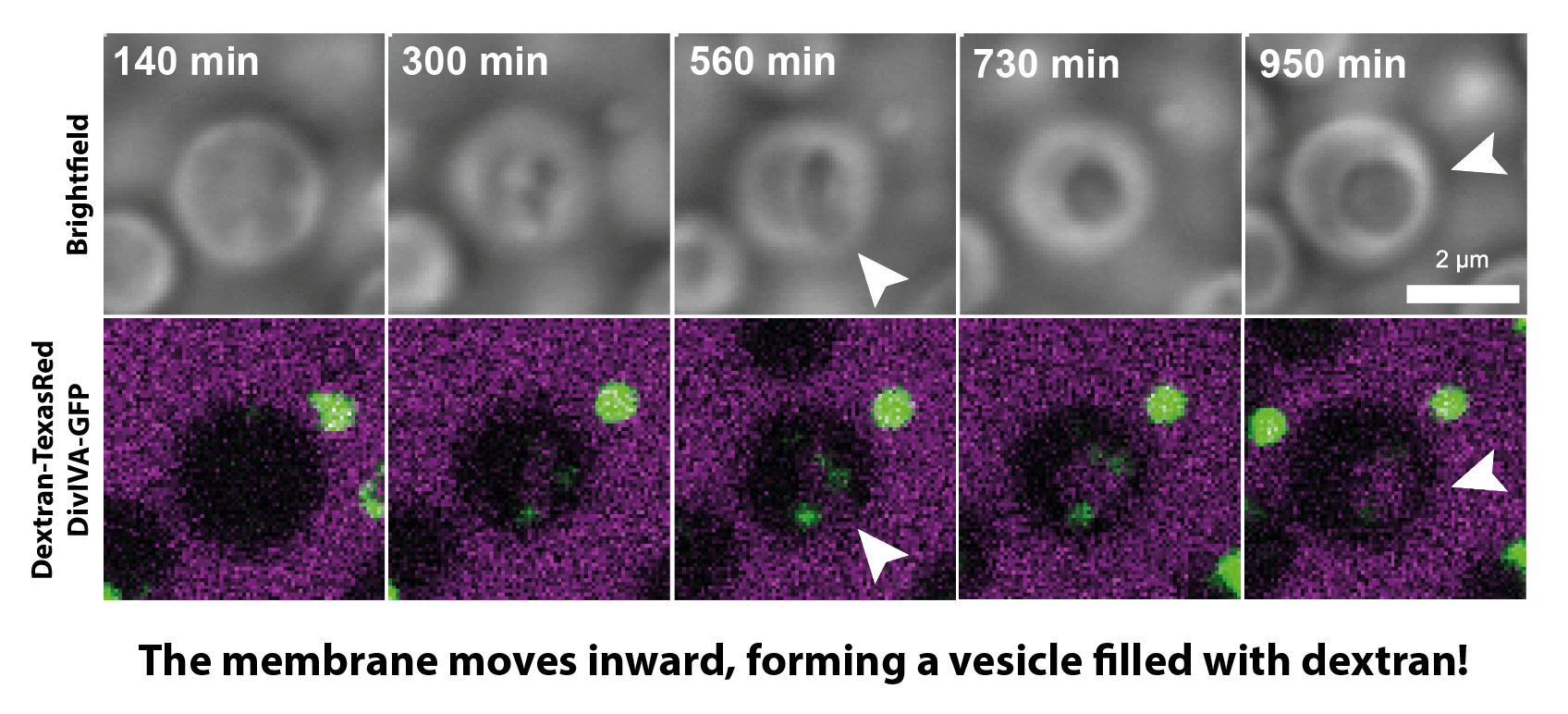
Images from a timelapse showing engulfment of fluorescently-labeled Dextran (magenta) by an L-form cell, resulting in vesicle formation.
Zooming in on the details
As there are limits to the detail we could see with our fluorescence microscope, we teamed up with electron microscopy experts. Using a state-of-the-art technique, called ‘FIB-SEM’ (Focused Ion Beam – Scanning Electron Microscope), we imaged the cells in high-resolution to see the vesicle in more detail (one pixel in the image represented five nanometer, around a billionth meter!). This was possible without using staining techniques, so we could look at cells as close to their normal morphology as possible. We could see details of the cells that we never saw before, such as complexes of vesicles and protrusions from the cell membrane. This also showed us that the membrane of L-forms is very pliable. This perhaps relates to their high membrane fluidity (a measure for the viscosity of the cell membrane). This would likely not have been possible if the cells still had a rigid cell wall!
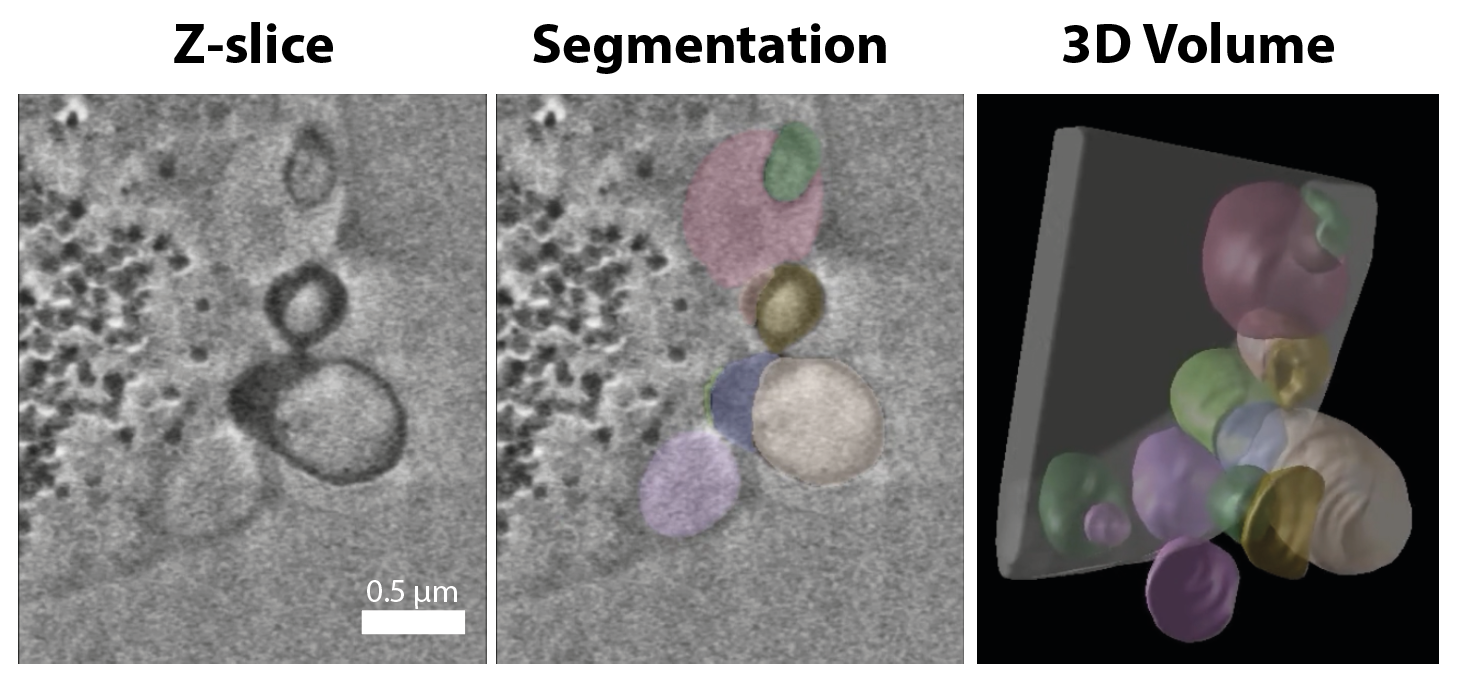
FIB-SEM image zoomed in on the cell membrane showing multiple vesicles and protrusions (left). Using a segmentation approach that assigns a color to each vesicle (middle), a 3D reconstruction was made to visualise the vesicle organisation in the L-form cell (right).
So far so good, but after engulfing DNA in a vesicle, it has to be released in the cytosol to be of value for the cell. Interestingly, imaging cells overnight showed that some of these internal vesicles disappeared from one image to the next. It is therefore likely that these vesicles were degraded thereby releasing DNA or other contents are in the cytoplasm, although we do not yet understand how this works.
Linking L-forms to fossils
Back to the early lifeforms. Is there any evidence that these cells were able to make such internal vesicles? That is of course difficult to prove. What we do know is that some microfossils from the Archaean Eon have similarities to bacteria without a cell wall, and indeed seem to contain internal vesicle structures5. Perhaps this endocytosis-like process could have helped these bacteria to take up external DNA or even food. Indeed, this robust uptake mechanism could have also worked to take up nutrients from the environment when no designated transporters were available yet.
Studying L-forms is not only relevant to understand the past, but also to better understand the plasticity of modern bacteria. We know that many, if not all bacteria, can readily switch to an L-form state. Our work shows that without a cell wall, L-forms can acquire new genes, for instance conferring resistance to antibiotics. By studying L-forms, we hope to develop effective intervention strategies to mitigate these potential risks, particularly for pathogenic bacteria that exploit this putative ancient mechanism of DNA uptake.
Publication:
Kapteijn, R., Shitut, S., Aschmann, D. et al. Endocytosis-like DNA uptake by cell wall-deficient bacteria. Nat Commun 13, 5524 (2022). https://doi.org/10.1038/s41467-022-33054-w
References
- Errington, J. L-form bacteria, cell walls and the origins of life. Open Biol. 3, 120143 (2013).
- Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465-1468 (1990).
- Cossart, P. & Helenius, A. Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect. Biol. 6, (2014).
- Elkin, S. R., Lakoduk, A. M. & Schmid, S. L. Endocytic pathways and endosomal trafficking: a primer. Wien. Med. Wochenschr. 166, 196-204 (2016).
- Kanaparthi, D. et al. On the nature of the earliest known lifeforms. bioRxiv, 456462 (2021).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in