One step closer to preventing invasive E. coli disease, including sepsis and bacteremia.
Published in Microbiology, Pharmacy & Pharmacology, and Immunology

What is invasive E. coli disease?
When we hear “E. coli”, many think of harmless gut bacteria or sometimes food poisoning/diarrhea. However, pathogenic strains of this common bacterium are far more dangerous and can cause infections outside the intestinal tract. These strains, known as Extraintestinal Pathogenic E. coli (ExPEC), are the most common gram-negative bacterial pathogen in humans.1 They can cause invasive E. coli disease manifesting as bacteremia (bacteria in the blood), sepsis (an extreme immune response to infection), and septic shock (a life-threatening condition where the body’s response to infection causes injury to its own tissues and organs). Additionally, E. coli is a leading cause of urinary tract infections (UTIs), such as cystitis/bladder infections or pyelonephritis/kidney infections.1,2
Each year, ExPEC causes ~10 million infections in the United States alone.3 In US community-based populations aged ≥65 years, invasive E. coli disease incidence is estimated to be ~150 cases/100,000, which is three times higher than the incidence among all adults.1
Why do we need to prevent invasive E. coli disease?
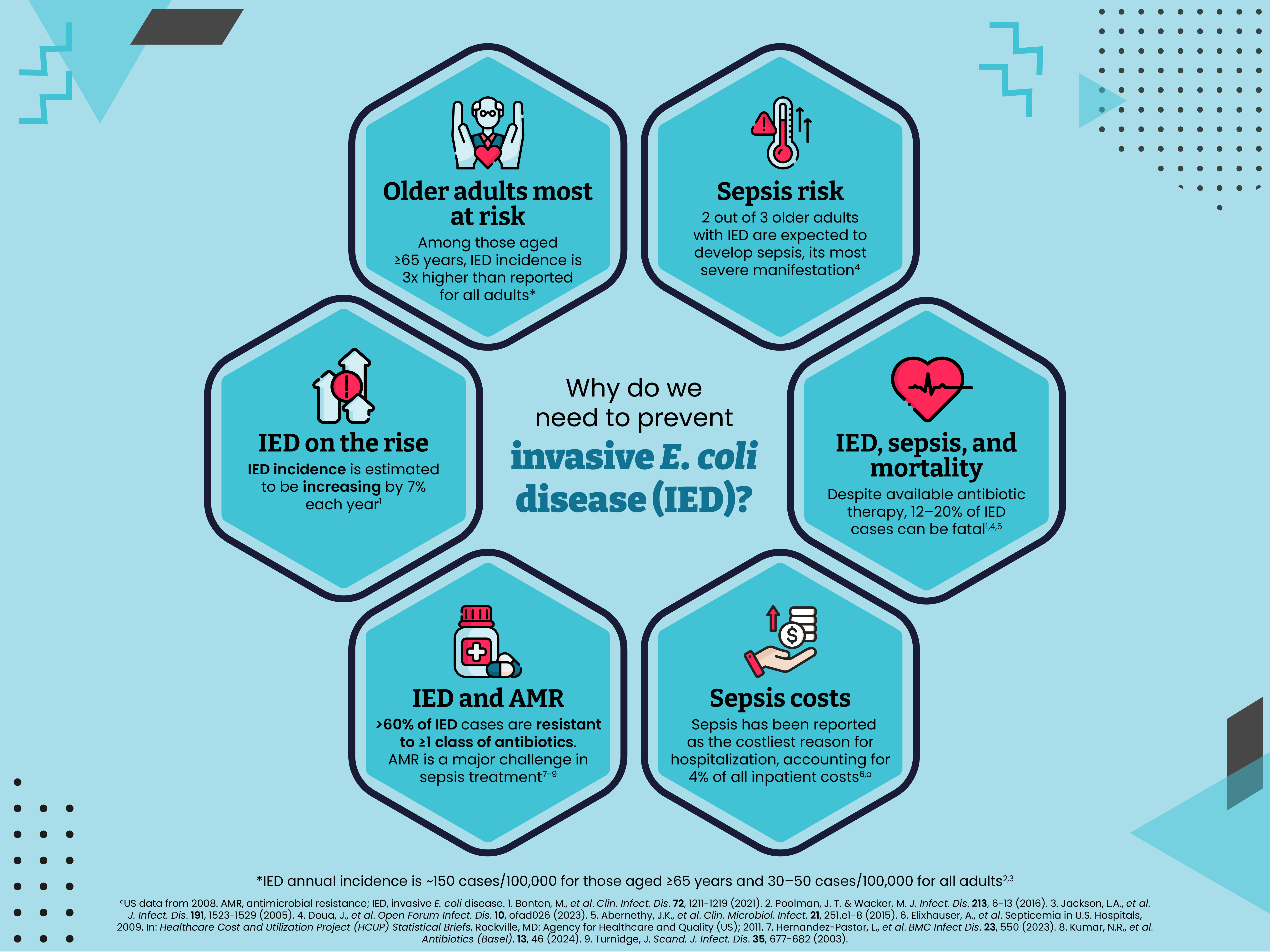
Nearly two-thirds of older adults with invasive E. coli disease are expected to develop sepsis, its most severe manifestation.2 Sepsis can lead to tissue damage, organ failure and potentially death.4 In 2001, ~40,000 deaths were caused by sepsis associated with invasive E. coli disease in the United States alone.3 In 2008, sepsis was the costliest reason for hospitalization in the United States, accounting for 4% of all inpatient costs.5 Using data from the Global Burden of Diseases, Injuries, and Risk Factors Study, there were 48.9 million cases of sepsis and 11.0 million sepsis-related deaths worldwide in 2017, representing 19.7% of all global deaths.6 In the same year, the World Health Organization published a resolution recognizing sepsis as a global health priority.7
Almost all patients with invasive E. coli disease will require hospitalization and around one-third of patients will be admitted to the intensive care unit.8,9 In a recent US study, the average time patients were hospitalized for was ~7 days, with 38% being readmitted within just one month of discharge.8 All-cause mortality rates of 12–20% have been reported among patients with invasive E. coli disease.2,10,11 Invasive E. coli disease has also been associated with considerable long-term effects on quality of life, with approximately one-third of patients transferred to a skilled nursing or intermediate care facility after hospital discharge.3,8
The incidence of invasive E. coli disease is estimated to be increasing by 7% each year and, despite available antibiotic therapy, mortality from these infections remains high.2,10,12 E. coli is the most common pathogen responsible for antimicrobial resistance (AMR)-associated morbidity and mortality.10,11,13,14 Antimicrobial-resistant E. coli strains are commonly associated with treatment failure, increased length of hospital stay, and potentially higher mortality.1,15 In a recent global study, the Antimicrobial Resistance Collaboration estimated there were 4.95 million deaths associated with bacterial AMR in 2019, of which 1.27 million were directly attributable to bacterial AMR. This study identified E. coli as the leading causative pathogen, found to be responsible for 219,000 deaths attributable to bacterial AMR.13 Recent US data indicate that 62% of invasive E. coli disease cases are associated with resistance to ≥1 class of antibiotics, while 34% demonstrate resistance to ≥3 classes of antibiotics.9
Emerging resistance to first-line treatment options among ExPEC strains is proving to be a significant barrier to invasive E. coli disease treatment leading to increased hospitalization and mortality on a global scale.1
What efforts are underway to develop a vaccine against invasive E. coli disease?
Despite available treatment options, the clinical and economic impact of invasive E. coli disease remains substantial, and novel preventive strategies (including vaccines) are needed. In recent years, significant progress has been made toward developing a vaccine against invasive E. coli disease. The O-antigen—an important virulence factor for E. coli—has been identified as a promising vaccine target.1 Vaccine candidates that contain a combination of O-antigens commonly associated with invasive E. coli disease have been shown to instigate an immune response with no safety concerns identified in early studies.16,17 Our paper published in June 2024 reports the safety and immunogenicity of a bioconjugate vaccine containing O-antigen polysaccharides from 10 prevalent ExPEC serotypes. This vaccine was evaluated in older adults with a history of UTIs—a population known to have a significantly high risk for invasive E. coli disease—and was shown to elicit a robust immune response and acceptable safety profile.18 A phase 3 clinical trial (NCT04899336) is evaluating a similar vaccine composition in adults aged ≥60 years who have had a UTI in the last two years.
What could the future look like with a vaccine against invasive E. coli disease?
Ultimately, a future with an effective vaccine against invasive E. coli disease could significantly reduce disease burden, the incidence of sepsis, antibiotic resistance, and associated healthcare costs. A vaccine could also improve overall public health outcomes and contribute to healthier aging.
References
1 Poolman, J. T. & Wacker, M. Extraintestinal Pathogenic Escherichia coli, a Common Human Pathogen: Challenges for Vaccine Development and Progress in the Field. J. Infect. Dis. 213, 6-13 (2016).
2 Doua, J. et al. Epidemiology, Clinical Features, and Antimicrobial Resistance of Invasive Escherichia Coli Disease in Patients Admitted in Tertiary Care Hospitals. Open Forum Infect. Dis. 10, ofad026 (2023).
3 Russo, T. A. & Johnson, J. R. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 5, 449-456 (2003).
4 Bode, C., Weis, S., Sauer, A., Wendel-Garcia, P. & David, S. Targeting the host response in sepsis: current approaches and future evidence. Crit. Care. 27, 478 (2023).
5 Elixhauser, A., Friedman, B. & Stranges, E. Septicemia in U.S. Hospitals, 2009. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare and Quality (US); 2011.
6 Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 395, 200-211 (2020).
7 Reinhart, K. et al. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N. Engl. J. Med. 377, 414-417 (2017).
8 Hernandez-Pastor, L. et al. Economic burden of invasive Escherichia coli disease among older adult patients treated in hospitals in the United States. J. Manag. Care Spec. Pharm. 29, 873-883 (2023).
9 Hernandez-Pastor, L. et al. Clinical burden of invasive Escherichia coli disease among older adult patients treated in hospitals in the United States. BMC Infect. Dis. 23, 550 (2023).
10 Bonten, M. et al. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin. Infect. Dis. 72, 1211-1219 (2021).
11 Rhee, C. et al. Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated With Inadequate and Broad-Spectrum Empiric Antibiotic Use. JAMA Netw. Open. 3, e202899 (2020).
12 Abernethy, J. K. et al. Thirty day all-cause mortality in patients with Escherichia coli bacteraemia in England. Clin Microbiol Infect. 21, 251 e251-258 (2015).
13 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 399, 629-655 (2022).
14 Weerdenburg, E. et al. Global Distribution of O Serotypes and Antibiotic Resistance in Extraintestinal Pathogenic Escherichia coli Collected From the Blood of Patients With Bacteremia Across Multiple Surveillance Studies. Clin. Infect. Dis. 76, e1236-e1243 (2023).
15 Mark, D. G. et al. Third-Generation Cephalosporin Resistance and Associated Discordant Antibiotic Treatment in Emergency Department Febrile Urinary Tract Infections. Ann. Emerg. Med. 78, 357-369 (2021).
16 Huttner, A. et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 17, 528-537 (2017).
17 Frenck, R. W., Jr. et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial. Lancet Infect. Dis. 19, 631-640 (2019).
18 Fierro, C. A. et al. A randomized phase 1/2a trial of ExPEC10V vaccine in adults with a history of UTI. NPJ Vaccines. 9, 106 (2024).
Medical writing support was provided by Amy Bray of Eloquent Scientific Solutions, and funded by Janssen Research & Development, LLC.
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in