Optical imaging of single protein size, charge, mobility and binding
Published in Electrical & Electronic Engineering

Written by: Guangzhong Ma and Shaopeng Wang
Biodesign Center for Bioelectronics and Biosensors, Arizona State University, Arizona 85287, USA
Gel electrophoresis and Western blot are the workhorse techniques for protein separation and identification in research labs. The separation in gel is based on the size, charge and mobility difference of proteins, and the following identification step is achieved by antibody binding. These processes require a certain amount of protein sample, otherwise the protein cannot be resolved in the gel. Inspired by these traditional protein detection methods and wishing to improve the sensitivity at the same time, we developed a single-molecule version of the protein analysis technique. As illustrated in Figure 1, we tethered single protein molecules to a surface with polymer linkers and drove the molecules into oscillation with an electric field. The tethered proteins were placed in an evanescent field and scattered light. By analyzing the scattering patterns, the oscillation amplitude of each individual protein can be extracted with nanometer precision. From these parameters, the size, charge, and mobility are derived.
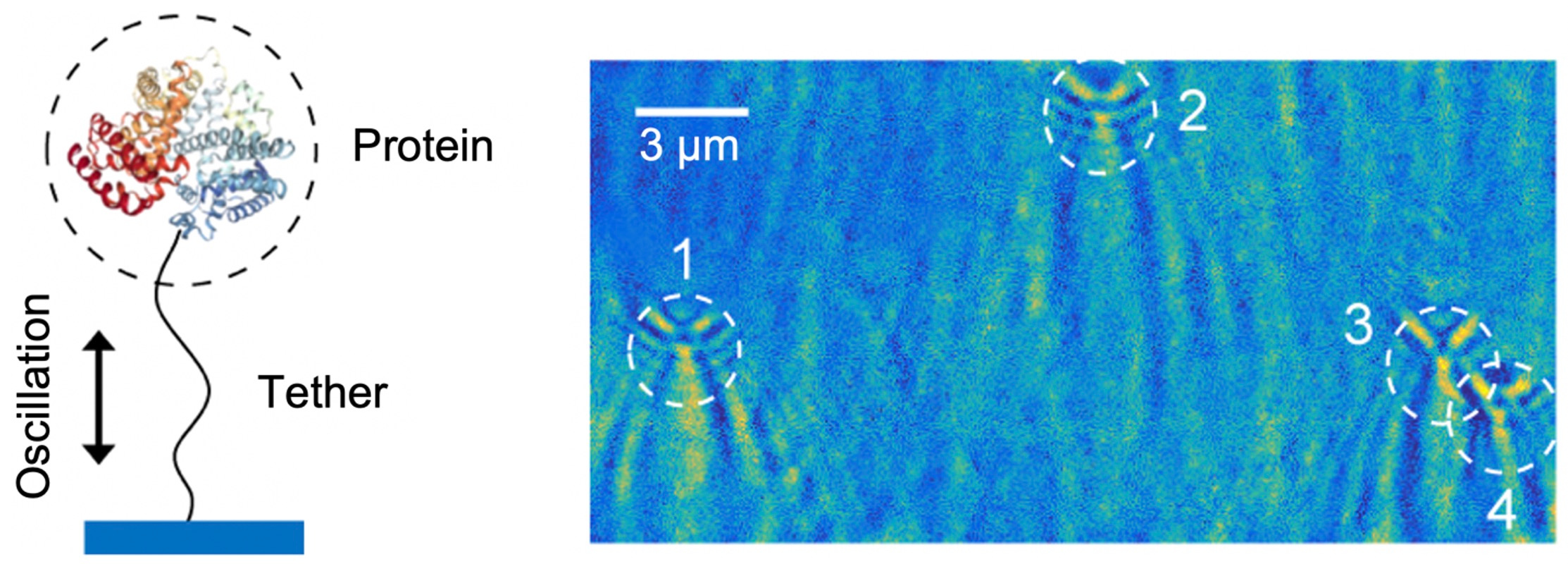
The oscillation idea was proposed by Dr. NJ Tao 10 years ago. The very first proof-of-concept experiment was measuring the charge and height of free micrometer-sized particles near the surface using surface plasmon resonance (SPR) imaging. The particle height can be precisely probed by the evanescent field in SPR. However, the determination of charge is confused by particle Brownian motion, especially when the particle size goes down. The free particles can also easily diffuse away. These problems were later solved by tethering the particle with a soft molecular linker. To further reduce the noise, periodical potentials were applied to the particles and fast Fourier transform (FFT) was used to extract the modulated signal while rejecting the Brownian noises. These improvements enabled us to measure the charge of nanoparticles with sub-elementary charge accuracy.
As the measurable size and charge became smaller and smaller, we thought it was possible to measure molecules as tiny as proteins. We first tried to fabricate PEG tethered proteins on gold surface and oscillated them the same way as we did for the particles, but this didn’t work, because the weak protein oscillation signal was obscured by the SPR response to the double-layer charging on the gold surface. We tried many different experimental conditions to reduce the charging and enhance the oscillation, with no success. We finally gave up gold and SPR and tried indium tin oxide (ITO) coated glass slide instead. ITO worked wonderfully for reducing the charging background and enabled us to visualize the oscillation patterns. It was not easy to confirm the patterns were due to single proteins, so we performed lots of control experiments: calibration, SEM, AFM, antibody detection… and eventually convinced ourselves that the signals were indeed from single proteins.
The discovery took 10 years from the initial idea to the final outcome. However, after finishing the paper, our beloved colleague and friend Dr. NJ Tao unfortunately passed away this spring. This work would not be done without his guidance and support. We dedicate this work to the memory of Dr. NJ Tao. A memorial website has been setup for him: https://www.njtaoasu.org
Link to the full paper: https://www.nature.com/articles/ s41467-020-18547-w
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in