Out Of The Blue, It Came To Me
Published in Microbiology, Protocols & Methods, and Cell & Molecular Biology
Scientific discovery does not always follow a straight line. Sometimes, it hits you when you least expect it—like a wave.
This story began not with a bold hypothesis, but with colony PCR. We were using a protocol picked up years earlier from a Harvard Medical School rotation student. Unlike the standard approach—adding colonies directly to the PCR mix—we first resuspended the cells in sterile water. It was a small procedural tweak that kept the bacteria viable for hours and made our workflow more flexible. At the time, it felt like a convenience. In hindsight, it changed everything.
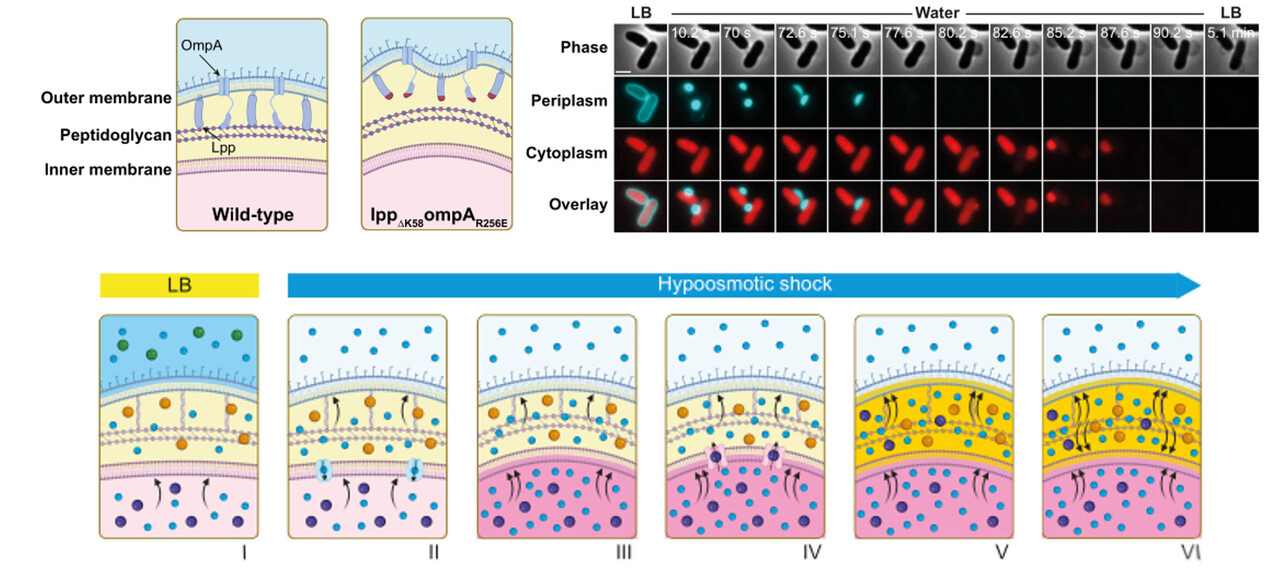
The surprise came when the very mutants we had been trying to generate (the “good” clones) consistently failed to grow after the water resuspension step. These were the strains with disrupted connections between their outer membrane and the peptidoglycan layer. Only the “bad” clones—those that had not acquired the second mutation—were able to survive for hours in distilled water.
We wanted to see with our own eyes how these cells were dying. Rather than the explosive bursting one might expect under osmotic stress, we observed a more gradual process. The first sign was a local detachment of the outer membrane, followed by the sequential release of periplasmic and cytoplasmic contents. Surprisingly, the cells maintained their overall shape throughout the water shock; there was no obvious rupture or collapse of the envelope.
These puzzling observations did not fit our assumptions. Like most microbiologists, we had internalized the textbook view: that the peptidoglycan layer is the primary structure preventing osmotic lysis in Gram-negative bacteria. But here we had cells with an intact peptidoglycan wall—yet they were dying quickly in pure water. The disruption was not in the wall itself, but in the way it was connected to the outer membrane. We could have easily dismissed the result as an experimental glitch and changed our PCR protocol. But something about it did not sit right. Our gut told us there was more to the story—and that instinct, more than any hypothesis, is what pushed us to keep digging.
It forced us to unlearn what we thought we knew. The textbook model, elegant in its simplicity, was missing a key part of the picture. If peptidoglycan alone were sufficient for osmotic resistance, these double mutants should have survived. They did not.
Mechanosensitive channels were already known to play a role in osmoprotection, releasing solutes from the cytoplasm to the periplasm under hypoosmotic stress. But they had rarely been considered in the broader architectural context of the bacterial envelope. This led us to re-examine the periplasmic space with fresh eyes. It is often portrayed as a passive zone—just the space between the membranes—and many still assume it is an open environment, freely exchanging solutes with the external medium. But that view is incomplete. The periplasm is a true compartment: confined, structured, and chemically distinct, with its own osmotic pressure, counter-balancing the cytoplasmic turgor. At one point, we even proposed a term for its contents—the perisol—in analogy with the cytosol, to emphasize that this space holds more than we tend to acknowledge.
It took time—and a few more unexpected results—but we eventually arrived at a more holistic view of the bacterial cell envelope. In this framework, the peptidoglycan is no longer the sole hero of osmotic resistance. Instead of acting as an autonomous wall, we began to see it more as a scaffold—one that provides shape and rigidity but depends on its integration with the rest of the envelope to function effectively under stress. Osmoprotection emerges from a larger, coordinated system that includes outer membrane tethering, periplasmic confinement, and responsive membrane channels. Each component matters, and it is their cooperation that keeps cells alive in the face of osmotic shock.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in