Overcoming the low reactivity of biobased, secondary diols in polyester synthesis
Published in Chemistry

Plastic materials have become an indispensable part of modern life due to their tunable and light-weight properties, which is reflected in the production of nearly 400 million tons of virgin plastic in 2019 [1], around 99% of which are produced from fossil resources [2]. A shift away from fossil- to bio- (and CO2-)based materials has the potential to decrease the carbon footprint of the plastic industry [3]. Additionally, utilizing the inherent functionalization of bioderived monomers, new materials with improved properties can be obtained [4]. The comparatively highly oxygenated structures of above-ground biomass makes the synthesis of polymers with oxygen-based repeat units sensible. One example for this are polyesters, whose ester bond additionally enables closed-loop chemical recycling, which is another important aspect for a more sustainable plastic industry [5].
We set out to improve the applicability of the promising, glucose-derived monomer isosorbide in polyester synthesis. Despite decades of research on this monomer, it is still challenging to obtain high molecular weight polyester materials, especially when using isosorbide as the sole diol moiety [6]. This is due to the low reactivity of isosorbide’s secondary alcohol group, which prevents chain growth during the last, crucial stages of polyester synthesis. This leads to low molecular weight materials, which can not be used in most applications due to their brittleness.
In this work, we have discovered that the addition of a monofunctional aryl alcohol to diol and diacid monomers during esterification leads to the formation of oligomers with reactive aryl ester end groups. Fig. 1 shows this synthesis strategy applied to poly(isosorbide succinate), a promising glucose-derived polyester, which until now could not be synthesized with high enough molecular weights for thermoplastic applications.
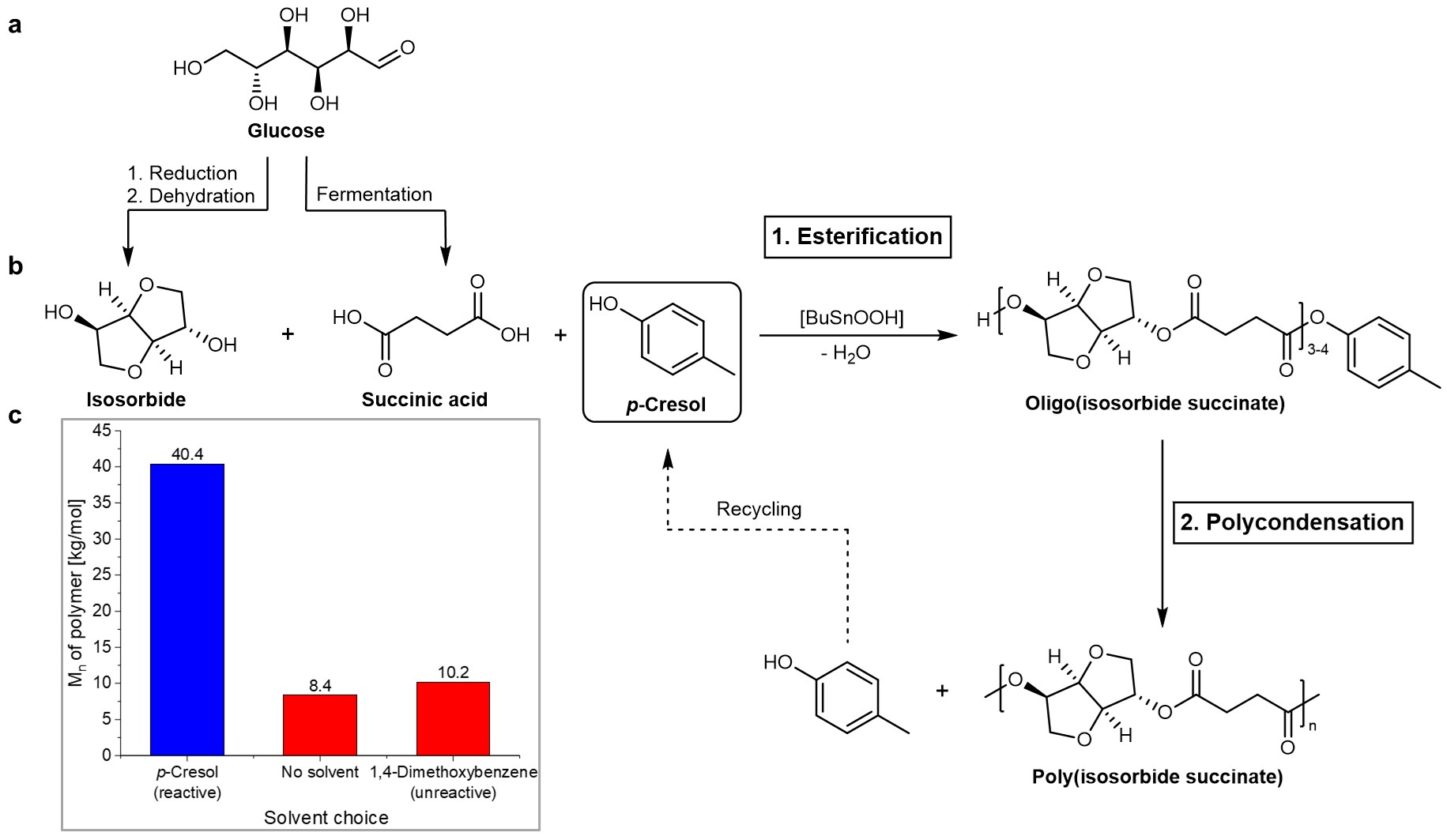
Figure 1. Synthesis of high molecular weight poly(isosorbide succinate) with an aryl alcohol. a Monomer synthesis from glucose, obtainable from either first or second generation biomass. b Synthesis of poly(isosorbide succinate) from succinic acid, isosorbide and p-cresol. c Poly(isosorbide succinate) molecular weights obtained with a reactive solvent (p-cresol), no solvent and an unreactive solvent (1,4-dimethoxybenzene) under comparable reaction conditions.
The aryl ester end groups obtained during esterification greatly facilitate the chain growth during polycondensation, leading to very high molecular weight polyesters based on unreactive diols like isosorbide and isomannide. The presented strategy enabled the synthesis of fully biobased polyesters like poly(isosorbide succinate) with molecular weights high enough to enable the characterization of the materials’ barrier and mechanical properties. Some of the synthesized materials’ properties were shown to be superior to established fossil-based materials. We also show that the synthesis strategy can be scaled from a 100 ml glass reactor to a 2 L stainless steel autoclave. This is especially interesting considering the commercial availability of all required reagents for the synthesis of poly(isosorbide succinate). The presented synthesis strategy could facilitate the commercial adaptation of fully biobased polyesters with promising material properties.
References:
1. EuropePlastics. Plastics - the Facts 2021. An analysis of European plastics production, demand andwaste data., https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (2021).
2. The global bio-based polymer market in 2019 – A revised view; nova Institute 2020; https://www.bioplasticsmagazine.com/en/news/meldungen/20200127-The-global-bio-based-polymer-market-in-2019-A-revised-view.php (Accessed 14-Nov-22).
3. Miller, S. A. Sustainable Polymers: Opportunities for the Next Decade. ACS Macro Lett 2, 550–554 (2013).
4. Zhu, Y., Romain, C. & Williams, C. K. Sustainable polymers from renewable resources. Nature 540, 354–362 (2016).
5. Häußler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021).
6. Weinland, D. H., van Putten, R.-J. & Gruter, G.-J. M. Evaluating the commercial application potential of polyesters with 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide) by reviewing the synthetic challenges in step growth polymerization. Eur Polym J 164, 110964 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in