Ozone — An Unexpected Ally Against Influenza?
Published in Public Health

A recently published study in Nature Communications demonstrated an inhibitory effect of ambient ozone (O3) on influenza activity in the USA over a 5-year period from 2010 to 2015. Using multiple methods for causal inference — Convergent Cross Mapping, Peter-Clark-momentary-conditional-independence plus (PCMCI+) graphical modelling, and Generalized Linear Model — the researchers were able to consistently detect a negative relationship between 1-week lagged O3 levels and influenza activity in the community.
This finding is intriguing given O3's reputation as an air pollutant and respiratory irritant. While previous laboratory studies have suggested that O3 at hundreds of ppb exhibits virucidal properties through its oxidizing power, another biological pathway of O3-primed host immunity against influenza infection is more plausible since the average level of daily maximum 8-hour O3 is less than 40 ppb in the study context. The current publication provides epidemiological evidence from observational time series data, lending support to those mechanistic hypotheses (Fig. 1).
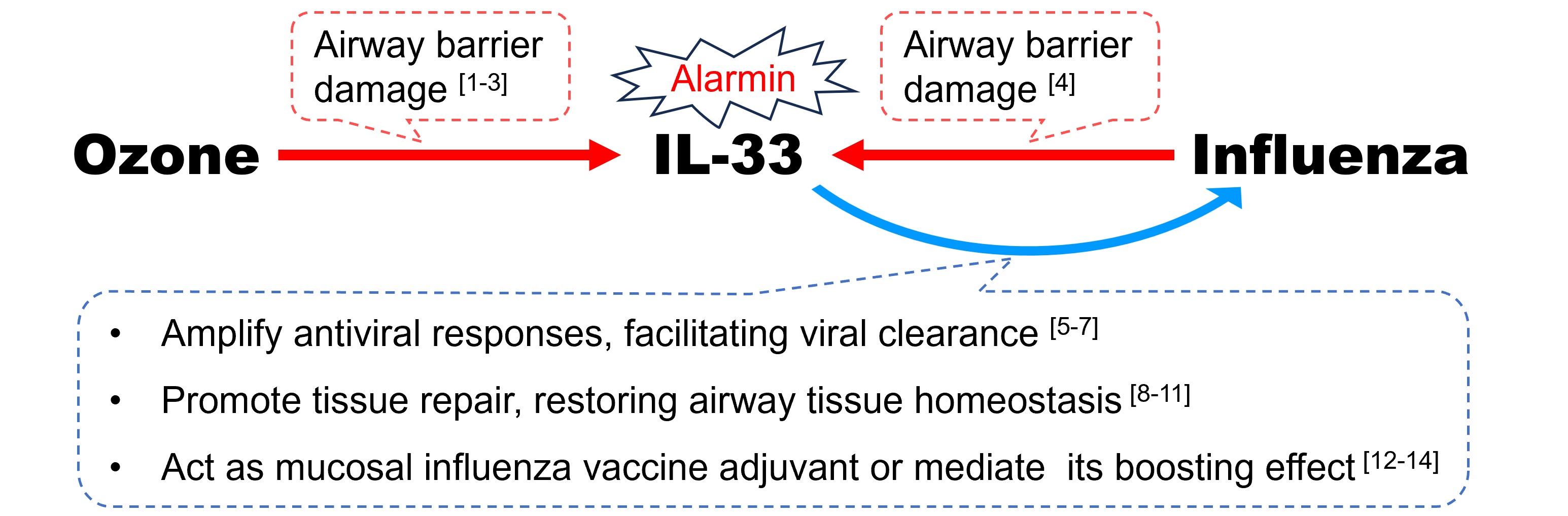
Figure 1. The hypothesis of ozone-elicited IL-33 conferring cross-protection against influenza
“O3 → IL-33”
Following O3 exposure, a myriad of immune responses is triggered and multiple interleukins (IL) are released from epithelial cells, macrophages, and other myeloid cells. Among them, IL-33, acting as an endogenous “alarmin” in response to airway barrier damage incurred by O3 [1,2], is endowed with pleiotropic and homeostatic functions orchestrating airway injury and repair [3].
“Influenza → IL-33”
Likewise, IL-33 is also highly expressed following invasion of influenza virus, playing a pivotal role of dynamic immune modulator during the course of infection [4]. It is plausible that O3-induced IL-33 in the cytokine milieu is involved in an immune crosstalk assisting human defense against influenza.
“IL-33 → Influenza”:
-
- Amplify antiviral responses, facilitating viral clearance.
In the setting of inflammation combating foreign antigen, over-expressed IL-33, signaled via its receptor ST2, can be redirected from the default type 2‑inducing capacity to augment type 1 immunity, amplifying antiviral CD8+ T cell and natural killer cell responses [5,6]. In mice models of influenza infection, exogenous IL-33 inoculation could enhance recruitment of dendritic cells (DCs), increase secretion of pro-inflammatory cytokine IL-12, and prime cytotoxic T-Cell responses, facilitating viral clearance [7].
-
- Promote tissue repair, restoring airway tissue homeostasis.
More importantly, IL-33 may protect against influenza by orchestrating Th1/Th2 paradigm and so maintaining a fine balance of pro-inflammatory pathogen clearance and anti-inflammatory tissue repair [8,9]. During the resolution phase of infection event, IL-33 can act on residential ST2-expressing group 2 innate lymphoid cells (ILC2s) as well as regulatory T (Treg) cells to restore airway tissue homeostasis, mediated at least partly by amphiregulin (AREG)-dependent repair of virus-damaged epithelium [10,11].
-
- Act as mucosal influenza vaccine adjuvant or mediate its boosting effect.
The hypothesis of O3-elicited IL-33 conferring cross-protection against influenza gains strength further from the evidence of its promising role as a mucosal vaccine adjuvant [12]. Exogenous IL-33 co-administered intranasally with recombinant influenza A hemagglutinin (rHA) induced significantly higher antigen (Ag)-specific plasma immunoglobulin G (IgG) and mucosal IgA antibody (Ab) levels as well as enhanced production of both Th1 and Th2–related cytokines, all of which resulted in better protective capacity of the vaccine [13]. Besides, endogenous IL-33 release, within 24 h, after administration of alum-adjuvanted nasal influenza vaccine, induced higher IgA Ab production via enhancing Ag presentation on DCs and promoting ILC2 activation [14]. These findings allude to possible parallels between adjuvanticity of nasally administered alum and ambient O3 exposure.
In this research work, the authors underscore the importance of "totality of evidence" by applying multiple analytical approaches with different underlying assumptions and potential biases to analyse the dynamic data. The fact that all three disparate methods converged on the same conclusion — the inhibitory effect of ozone on influenza — strengthens the robustness of the findings. Meanwhile, the authors note that these novel population-level findings also call for in-depth studies to elucidate the underlying biological mechanisms, so further bolstering the credibility of causal inference linking ozone exposure to reduced influenza activity. This study raises the intriguing possibility that air quality regulations targeting ozone levels could be optimized and leveraged as an additional tool in the arsenal against seasonal and pandemic influenza threats.
References:
[1] Yang, Qi, Moyar Q. Ge, Blerina Kokalari, Imre G. Redai, Xinxin Wang, David M. Kemeny, Avinash Bhandoola, and Angela Haczku. 2016. “Group 2 Innate Lymphoid Cells Mediate Ozone-Induced Airway Inflammation and Hyperresponsiveness in Mice.” The Journal of Allergy and Clinical Immunology 137 (2): 571–78.
[2] Mathews, Joel A., Nandini Krishnamoorthy, David Itiro Kasahara, Youngji Cho, Allison Patricia Wurmbrand, Luiza Ribeiro, Dirk Smith, Dale Umetsu, Bruce D. Levy, and Stephanie Ann Shore. 2017. “IL-33 Drives Augmented Responses to Ozone in Obese Mice.” Environmental Health Perspectives 125 (2): 246–53.
[3] Sokolowska, Milena, Valerie F. J. Quesniaux, Cezmi A. Akdis, Kian Fan Chung, Bernhard Ryffel, and Dieudonnée Togbe. 2019. “Acute Respiratory Barrier Disruption by Ozone Exposure in Mice.” Frontiers in Immunology 10 (September): 2169.
[4] Le Goffic, Ronan, Muhammad Imran Arshad, Michel Rauch, Annie L’Helgoualc'h, Bernard Delmas, Claire Piquet-Pellorce, and Michel Samson. 2011. “Infection with Influenza Virus Induces IL-33 in Murine Lungs.” American Journal of Respiratory Cell and Molecular Biology 45 (6): 1125–32.
[5] Bonilla, Weldy V., Anja Fröhlich, Karin Senn, Sandra Kallert, Marylise Fernandez, Susan Johnson, Kreutzfeldt Mario, et al. 2012. “The Alarmin Interleukin-33 Drives Protective Antiviral CD8+ T Cell Responses.” Science 335 (6071): 984–89.
[6] Baumann, Claudia, Weldy V. Bonilla, Anja Fröhlich, Caroline Helmstetter, Michael Peine, Ahmed N. Hegazy, Daniel D. Pinschewer, and Max Löhning. 2015. “T-Bet- and STAT4-Dependent IL-33 Receptor Expression Directly Promotes Antiviral Th1 Cell Responses.” Proceedings of the National Academy of Sciences of the United States of America 112 (13): 4056–61.
[7] Kim, Chae Won, Hye Jee Yoo, Jang Hyun Park, Ji Eun Oh, and Heung Kyu Lee. 2019. “Exogenous Interleukin-33 Contributes to Protective Immunity via Cytotoxic T-Cell Priming against Mucosal Influenza Viral Infection.” Viruses 11 (9).
[8] Villarreal, Daniel O., and David B. Weiner. 2014. “Interleukin 33: A Switch-Hitting Cytokine.” Current Opinion in Immunology 28 (June): 102–6.
[9] Molofsky, Ari B., Adam K. Savage, and Richard M. Locksley. 2015. “Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation.” Immunity 42 (6): 1005–19.
[10] Arpaia, Nicholas, Jesse A. Green, Bruno Moltedo, Aaron Arvey, Saskia Hemmers, Shaopeng Yuan, Piper M. Treuting, and Alexander Y. Rudensky. 2015. “A Distinct Function of Regulatory T Cells in Tissue Protection.” Cell 162 (5): 1078–89.
[11] Monticelli, Laurel A., Gregory F. Sonnenberg, Michael C. Abt, Theresa Alenghat, Carly G. K. Ziegler, Travis A. Doering, Jill M. Angelosanto, et al. 2011. “Innate Lymphoid Cells Promote Lung-Tissue Homeostasis after Infection with Influenza Virus.” Nature Immunology 12 (11): 1045–54.
[12] Williams, Clare M., Sreeja Roy, Danielle Califano, Andrew N. J. McKenzie, Dennis W. Metzger, and Yoichi Furuya. 2021. “The Interleukin-33-Group 2 Innate Lymphoid Cell Axis Represents a Potential Adjuvant Target To Increase the Cross-Protective Efficacy of Influenza Vaccine.” Journal of Virology 95 (22): e0059821.
[13] Kayamuro, Hiroyuki, Yasuo Yoshioka, Yasuhiro Abe, Shuhei Arita, Kazufumi Katayama, Tetsuya Nomura, Tomoaki Yoshikawa, et al. 2010. “Interleukin-1 Family Cytokines as Mucosal Vaccine Adjuvants for Induction of Protective Immunity against Influenza Virus.” Journal of Virology 84 (24): 12703–12.
[14] Sasaki, Eita, Hideki Asanuma, Haruka Momose, Keiko Furuhata, Takuo Mizukami, and Isao Hamaguchi. 2021. “Nasal Alum-Adjuvanted Vaccine Promotes IL-33 Release from Alveolar Epithelial Cells That Elicits IgA Production via Type 2 Immune Responses.” PLoS Pathogens 17 (8): e1009890.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in