
When, in late 2019, a new virus of the coronaviridae family materialized in the Hubei Province of central China, I paid little attention. Several months later this virus, now branded SARS-CoV-2, was rapidly spreading across borders. Stories of hospitals overwhelmed by sufferers of coronavirus disease, or COVID, were distressingly frequent. In March of 2020, the WHO affirmed the severity of the situation by declaring a global pandemic, though, as we well know, the tailspin was soon to become a nosedive.
In that same month, I remember with clarity the moment that I and my university housemates watched the UK’s first national lockdown being announced. It was here, as the injunction to ‘stay at home’ was grimly given, that I began to see the novelty and gravity of what was happening. This lockdown marked an unceremonious end to my university career (literally), which was followed by a stultifying few months much like anyone else’s. Things swiftly changed, however, with the opportunity to join the research team of prolific biochemist and infectious disease scientist Dr Katie Doores; stationed at King’s College London, I was soon to begin researching the infamous SARS-CoV-2 virus.

The Doores Research Group’s objective is to study adaptive immune responses to SARS-CoV-2 infection and vaccination. We assess the potency of serum antibodies by measuring their ability to bind to the virus’s spike protein and prevent infection. We take this further by determining their ability to neutralize different variants of the SARS-CoV-2 virus, that is, to block the virus from gaining entry into cells. These profiles are recorded over several months post-exposure, from which we plot a temporal antibody response and deduce antibody longevity.
The techniques we use to determine these qualities are the ELISA (enzyme-linked immunosorbent assay) and the neutralization assays. The former tells us how much antibody from a given plasma sample attaches to a given substrate (in this case, the spike protein). To do this, we immobilize the spike protein to a surface. We then add a diluted sample of a patient’s plasma, some of whose constituent antibodies attach to the immobilized spike protein. We then add another, wholly different antibody to the plasma’s IgG antibodies – this secondary antibody holds an enzyme which, when mixed with the right reagent, will produce a colour change in the solution: the magnitude of the colour change (measured by light absorption) indicates the amount of antibody that initially became attached to the immobilized spike protein.
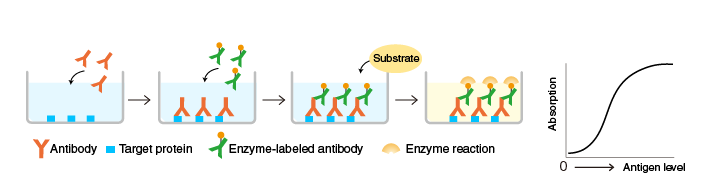
In conjunction with ELISA, we use the neutralization assay to determine how effectively a plasma sample stops the SARS-CoV-2 virus from infecting its target cells. We mix the virus and cells together, along with various concentrations of patient plasma, and incubate them for several days. We use an especially helpful type of virus which lets us know when it’s infected a cell – it does this by incorporating a gene into the cell which glows in certain conditions. This is the luciferase gene, responsible for the magnificent displays of bioluminescence observed in various species of firefly, jellyfish, and photobacteria. As the biblically-versed will recognize, its name comes from the eponymous fallen angel, literally the ‘light-bearer’; hence, the brighter the sample, the more infected cells are present within. This assay is particularly versatile, owing to its ability to measure a sample’s neutralizing capacity against several viral variants at once. These values can be compared to each other, and inferences made about key mutations from different variants and the roles these play in the tendency to be blocked by an antibody.
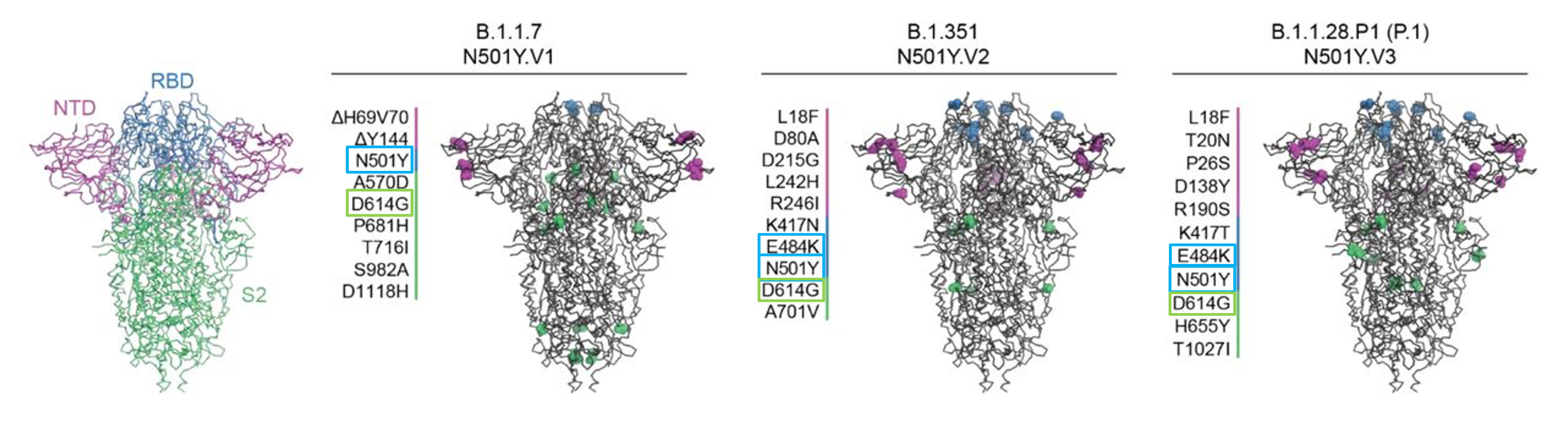
Figure 3b: Model of the SARS-CoV-2 spike protein with its main domains highlighted (left). Key mutations of 3 variants of concern (B.1.1.7, B.1.351, and P.1) are also shown (modified from Caniels et al., 2021, https://doi.org/10.1101/2021.05.26.21257441)
Together, these two assays paint a complete and detailed image of a person’s antibody response – either to a vaccination, or an infection, or both. From this we can infer the quality and breadth of protection induced by these various exposures. Factor in different time points, and not only do we then see how long this response lasts, but how it changes, or evolves, over time. Given the sudden and explosive advent of this virus and the limited information available on the durability of its antibody responses, this information is of paramount importance to the optimization of vaccines and maintenance of collective immunity. In fewer words, pretty promising stuff.
One of our recent articles (linked above) details how the antibody responses from wave 1 infections persist for almost a year after onset of symptoms. More saliently, we observed that while these patients were less able to neutralize the Alpha (B.1.1.7) and Beta (B.1.351) variants, and while the overall neutralizing capacity gradually decreased, the differences between neutralizing capacity against different variants also decreased, becoming more similar with time (illustrated below). I found this a thoroughly encouraging observation.
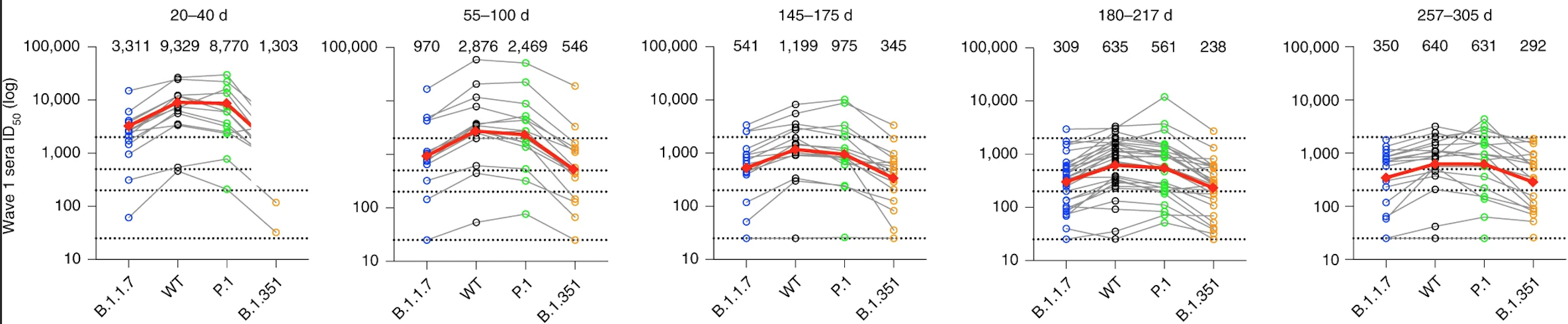
This observation is thought to be due to affinity maturation, a phenomenon first hypothesized in part by Niels Jerne and David Talmage and later expounded by Sir Frank Macfarlane Burnet. The process, occurring primarily in the lymph nodes, causes the ability of an antibody to bind to its target (its affinity) to increase over time. In a microcosmic version of Darwin’s theory of natural selection, B cells undergo an increased rate of mutation in their antibody genes – possibly up to a million times higher than normal. This produces a population of B cells with enormously varied antibody affinities. Specialized cells then select the B cells with higher-affinity antibodies, which go on to mature and proliferate, contributing to the evolution of a more potent neutralizing antibody pool. This wide breadth of response is crucial to long-term and widespread protection: a cross-neutralizing capacity that becomes more uniform over time suggests an ability to tolerate future emergences of different SARS-CoV-2 variants, hence the modest protection in many wave 1 patients against the notorious Delta (B.1.617.2) variant, which they had never encountered.
Our article gives reassurances about the durability of immunity, which has since assuaged my belated fear of the C-word. I hope that the findings of our article, and those of all articles being produced in this field, have the same reassuring effect on all who read them. Moreover, the resounding success of the UK’s vaccination programme has left me feeling far more positive about the situation than I did at the start of the year: the global nosedive seems to be manoeuvring an aerial U-turn. Though I definitely do not claim the right to speculate on the epidemiology with any authority, I feel highly optimistic about sharing a future with this novel coronavirus. That is, I look forward to collectively working towards an optimum balance between Panic! and Disco.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in