Persister cell phenotypes contribute to poor patient outcomes after neoadjuvant chemotherapy in PDAC
Published in Cancer

Pancreatic ductal adenocarcinoma (PDAC) is a deadly disease characterized by a high level of resistance against chemotherapy, immunotherapy, and targeted therapy. Despite the emergence of these newer latter forms of treatment the most effective current treatment of patients with PDAC in terms of prolonging survival remains surgical resection with some form of systemic cytotoxic therapy. Combination chemotherapy, especially FOLFIRINOX (oxaliplatin, irinotecan and 5-fluorouracil) and gemcitabine plus capecitabine or nab-paclitaxel have improved response rates and survival compared to monotherapies. However, even tumors that initially respond to chemotherapy usually become resistant within a matter of months in the advanced setting resulting in cancer progression and death in nearly all cases, or return after 1-2 years after surgical removal and subsequent chemotherapy ultimately causing death in most patients.
The mechanisms of primary and acquired PDAC resistance to chemotherapy are not yet well understood, but it is now clear that transcriptional tumor subtypes with different levels of chemotherapy resistance exist1,2. It has been difficult to study acquired resistance since tumor biopsies are usually only taken at the time of diagnosis, but not repeated longitudinally during the course of the disease – progression or ‘cure’.
In about 20-30% of PDAC patients, the primary tumor has not yet spread to other organs and can be resected (‘resectable’). An increasing proportion of patients undergoing resection are now pretreated with chemotherapy, either because the tumor was initially ‘unresectable’ and needed downstaging (with induction chemotherapy) or because the tumor was only ‘borderline resectable’ and treated with preoperative (‘neoadjuvant’) chemotherapy to improve survival3,4. Furthermore, induction chemotherapy can also lead to secondary resection in oligometastastic PDAC following a good tumor response with at least stable disease or a remission on re-staging after completing induction treatment3,5. This concept has only evolved with the availability of more effective chemotherapy protocols and advanced surgical techniques, and is currently under evaluation in a series of clinical studies3,6. The utility of this approach is highly dependent on the response of primary and metastatic lesions which underlines the clinical importance of understanding mechanisms of tumor resistance to enable the selection of the most effective therapy for individual patients. This is a major challenge right now.
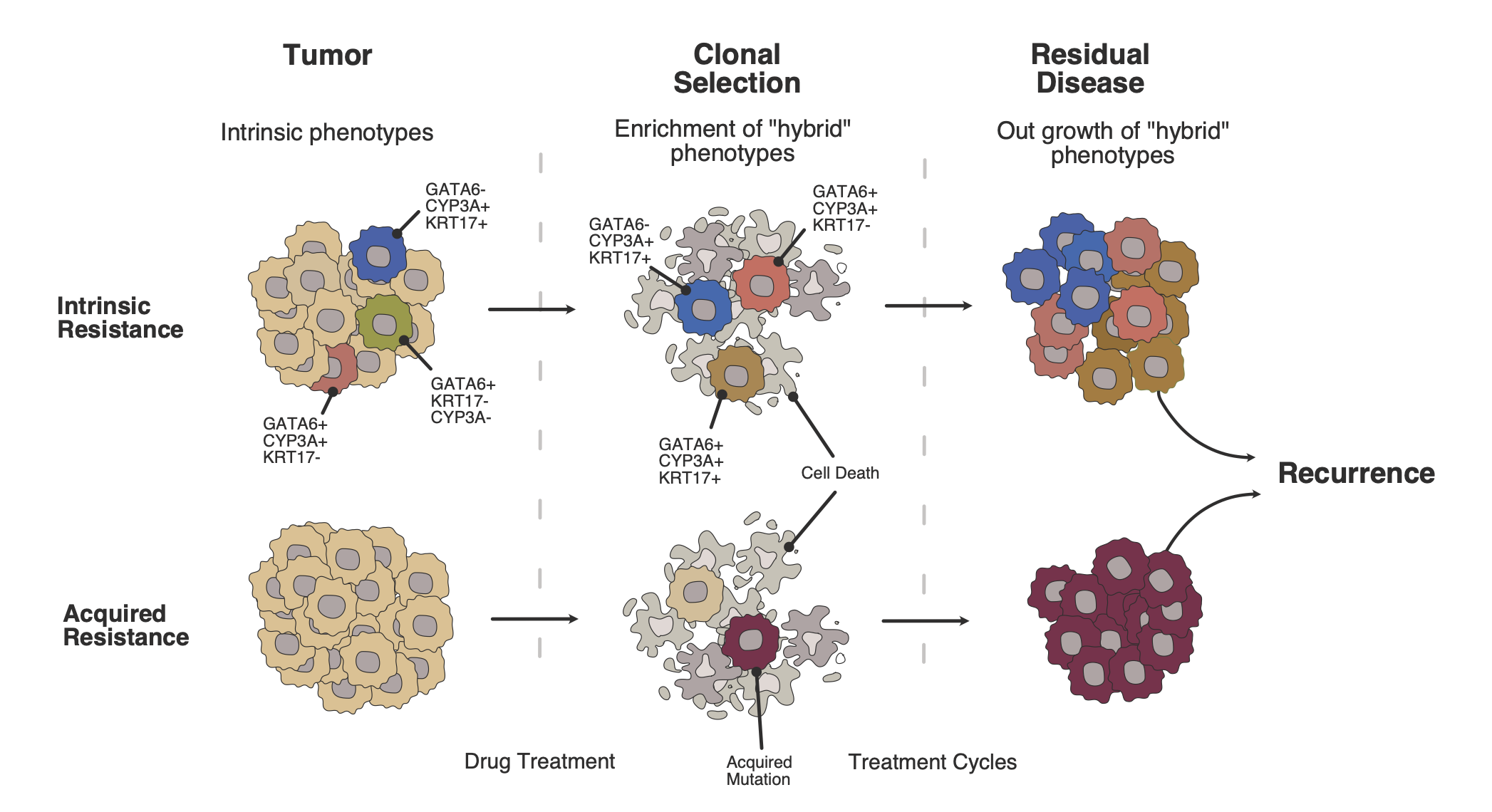
Resected tumors from patients pretreated with induction or neoadjuvant chemotherapy are a unique resource in which to study resistance mechanisms against chemotherapy. In this study, tumors treated with neoadjuvant chemotherapy and then resected were compared in detail with tumors resected upfront without previous chemotherapy. Pretreated and chemotherapy-naïve tumors differed distinctly at the molecular level. There was a shift from the Classical molecular subtype of tumors to the more chemo-resistant Basal-like tumor subtype. Moreover, ‘persister cells’ with molecular markers of both subtypes that also express a specific form of cytochrome P450 (CYP3A) were greatly enriched following pre-surgical treatment with FOLFIRINOX chemotherapy and were associated with a worse prognosis. Interestingly this was not found in tumors that had been treated with gemcitabine-based chemotherapy. Organoids derived from human PDAC tumors that over-expressed CYP3A also showed resistance to irinotecan (one of the components of the FOLFIRINOX regimen) with reduced conversion of irinotecan to the active metabolite SN38.
The results of this study are in line with previous findings that there is a continuous molecular spectrum of tumors between the ‘Classical’ and the ‘Basal’ transcriptomic subtypes2,7, and that there is tumor heterogeneity as well as great plasticity in response to chemotherapy2,8. Intra-tumoral CYP3A expression had already been described as a mechanism of PDAC resistance to paclitaxel and tyrosine kinase inhibitors9, and this mechanism was now shown to be an important factor driving the resistance of the persister cells to irinotecan. A study that tests CYP3A inhibition with cobicistat in parallel with gemcitabine and nab-paclitaxel is currently recruiting patients in Heidelberg (IntenSify study, NCT05494866).
A better understanding of chemotherapy resistance might assist the improvement of PDAC therapy in the future. Presently it is unclear how best to treat patients after neoadjuvant treatment and resection, since recurrence remains high, and survival is limited. In contemporary practice, patients just receive more of the same chemotherapy after the operation that they had before the resection. Molecular testing of the resected tumor currently has no role in the planning of adjuvant chemotherapy. An approach including the analysis of the persistent tumor cells to adapt the adjuvant therapy to the patient´s individual tumor might improve the efficacy chemotherapy in this setting.
Ideally, tissue samples before starting neoadjuvant therapy could serve as a resource for longitudinal comparison of molecular changes in the same patient and finally allow prediction of the development of certain resistance mechanisms and specify tumor biology at the time of diagnosis. This could be advantageous in stratifying patients prognostically not only for the correct choice of chemotherapy but help to select candidates for extended surgical approaches in locally advanced PDAC. Conversely, patients could also be deferred or excluded from extended surgical approaches where this is a highly aggressive systemic tumor phenotype. Even if there is, technically speaking, potential resectability, such patients with a very high likelihood of very early postoperative relapse could be better managed by systemic treatment – at least initially and then reviewed if performance status is maintained, by CT, circulating CA19-9, cell free DNA (cfDNA), and repeat tumor biopsy with sequencing. Whilst there is considerable interest in refining the methodology of liquid biopsies (such as circulating tumor cells, circulating tumor DNA, tumor extracellular vesicles, cfDNA, etc), these entities will not capture the full tumor phenotype as the stromal component, which is so important for resistance, is missing. Enhanced engineering methods are thus needed to increase the ease and speed of repeat tumor biopsy procedures.
Clinical studies accompanied by translational research will be necessary to explore these concepts with the aim of furthering individualized therapy aiming to improve the prognosis of PDAC patients. The European Study Group for Pancreatic Cancer (ESPAC) studies 6 (adjuvant, NCT05314998) and 7 (neoadjuvant) have been specifically designed to test the ternate concept of empirical staging and treatment, intrinsic and acquired tumor plasticity, and persister cell type enrichment, within the context of a (randomized) controlled setting.
Blog contributed by Christoph Springfeld, Thilo Hackert, Markus W. Büchler and John P. Neoptolemos.
REFERENCES
- Zhou, X. et al. Clinical Impact of Molecular Subtyping of Pancreatic Cancer. Front Cell Dev Biol 9, 743908 (2021). https://doi.org:10.3389/fcell.2021.743908
- Bailey P, et al. Refining the Treatment of Pancreatic Cancer From Big Data to Improved Individual Survival. Function (Oxf). 24(3):zqad011. (2023). https://doi: 10.1093/function/zqad011
- Springfeld, C. et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol 20, 318-337 (2023). https://doi.org:10.1038/s41571-023-00746-1
- Ghaneh P, et al. European Study Group for Pancreatic Cancer. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 8(2):157-168. (2023) https://doi: 10.1016/S2468-1253(22)00348-X
- Hank, T. et al. Oncological Outcome of Conversion Surgery After Preoperative Chemotherapy for Metastatic Pancreatic Cancer. Ann Surg. 277(5):e1089-98 (2022) https://doi: 10.1097/SLA.0000000000005481.
- Conradi, L. et al. Oncological surgery in the interdisciplinary context-On the way to personalized medicine. Chirurg. 93(3):234-241. (2022) https://doi: 10.1007/s00104-022-01614-x
- Chan-Seng-Yue, M. et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat Genet 52, 231-240 (2020). https://doi.org:10.1038/s41588-019-0566-9
- Raghavan, S. et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell 184, 6119-6137 e6126 (2021). https://doi.org:10.1016/j.cell.2021.11.017
- Noll, E. M. et al. CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma. Nat Med 22, 278-287 (2016). https://doi.org:10.1038/nm.4038
Follow the Topic
-
Nature Cancer

This journal aims to provide a unique forum through which the cancer community will learn about the latest, most significant cancer-related advances across the life, physical, applied and social sciences.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in