Photoinduced ynamide structural reshuffling and functionalization
Published in Chemistry
The concept of radical chemistry on ynamides has recently drawn the attention of synthetic organic chemists to the construction of various N-heterocyclic compounds. Nevertheless, the ynamide-radical chemistry remains a long-standing challenge for chemists due to its high reactivity, undesirable byproducts, severe inherent regio- and chemoselective problems. Importantly, the ynamide C(sp)-N bond fission remains an unsolved challenge. The well-established strategies for ynamides with help of transition-metal-catalyzed (metal carbene)/Bronsted acid-mediated (keteniminium ion) intermediates, which are then trapped by various nucleophiles/electrophiles to yield various difunctionalization products or N-heterocyclic compounds; these intermediates have been studied by various research groups (Liu, Hashmi, Ye, Sahoo, Gandon, and numerous other groups) (Fig. 1a).1-2 In addition, metal/oxidant/photocatalyst-induced radical addition to the α/β-carbons of ynamides leads to a mixture of E/Z isomers in the products (Fig. 1b).3-5 Recently, professors Gandon, Sahoo and coworkers observed an intermolecular radical-triggered reactivity of alkynes vs. ynamides in yne‐tethered ynamides with sulfur radicals under traditional reaction conditions (Fig. 1c).6-7 Importantly, selective intermolecular radical-triggered ynamide bond fission and structural reshuffling have remained unanswered challenges in ynamide chemistry until now.
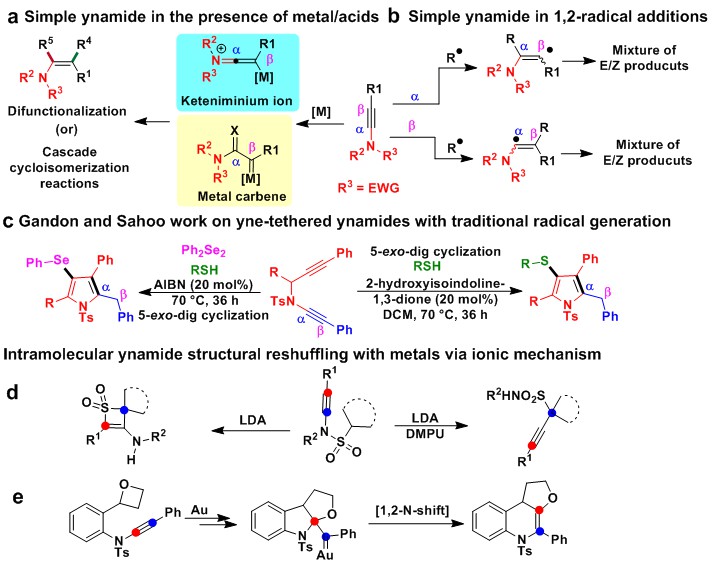
Fig. 1 Previous literature and background for reaction development. a Simple ynamide in a metal/Lewis acid. b Regioselective radical addition on α,β-carbons. c Radical chalcogen-triggered cascade reactions on yne-tethered ynamides. d Intramolecular ynamide structural reshuffling with lithium diisopropylamine (LDA) via an ionic pathway. e Intramolecular ynamide structural reshuffling with gold-catalyst.
Structural reshuffling is a process involving multiple-bond fission and new bond formation for molecular skeleton reassembly. However, ynamide structural reshuffling has scarcely been reported. In 2020, Cui and coworkers reported unusual, ionic divergent intramolecular structural reshuffling of ynamides aided by lithium diisopropylamine (LDA) to produce thiete sulfones, while the additional use of 1,3-dimethyl-tetrahydropyrimidin-2(1H)-one (DMPU) altered the process to produce propargyl sulfonamides (Fig. 1d).8 Very recently, Li and coworkers reported intramolecular ionic gold-catalyzed, 1,2-N-migration of ynamides via 1,1-carboalkoxylation in an atom-economical synthesis of tetrahydrofuran-fused 1,4-dihydroquinolines (Fig. 1e).9
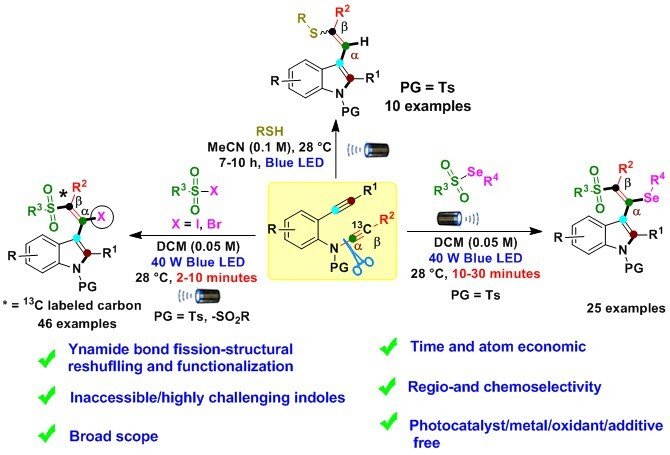
Despite their advantages, these limited existing radical strategies require expensive metals/photocatalysts, oxidants, longer reaction times, harmful waste production, and a lack of atom economy. Importantly, selective intermolecular radical-triggered ynamide bond fission and structural reshuffling have remained unanswered challenges in ynamide chemistry until now. In this work, we observe radical trigger regio- and chemoselective intermolecular ynamide C(sp)-N bond fission, structural reshuffling and functionalization producing synthetically inaccessible/challenging substituted indole derivatives (Fig. 2). We are utilizing 2-alkynyl-ynamides with divergent radical precursors to prepare chalcogen-substituted indoles derivatives via the formation of multiple bonds (N-C(sp2), C(sp2)-C(sp2), C(sp2)-SO2R/C-SR, and C-I/C-Se/C-H) in a rapid transformation that occurs under mild reaction conditions with excellent step/atom economy (Fig. 2).
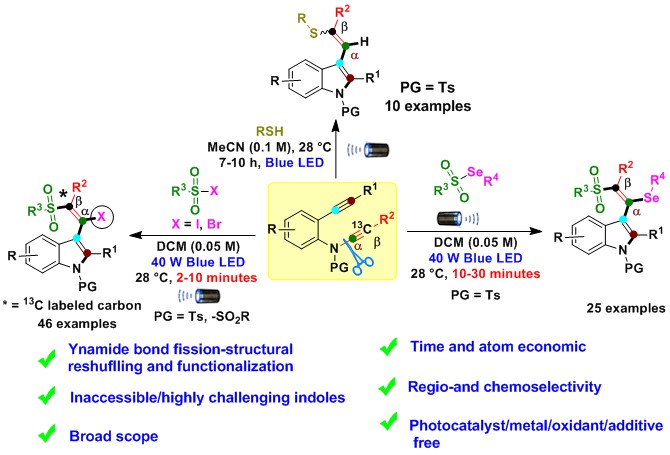
Fig. 2 Our photoinduced radical diversification strategy for inaccessible/challenging highly substituted indoles.
Indoles are favorable structural motifs that appear in numerous marketed drugs, the pharmaceutical industry, drug discovery, material chemistry and numerous other fields, including recent therapeutic leads. Thus, the construction of inaccessible/challenging indole ring system was a goal for altering the native indole cores, thus enabling access to chalcogen-substituted indoles as potential building blocks for drug discovery in the future.
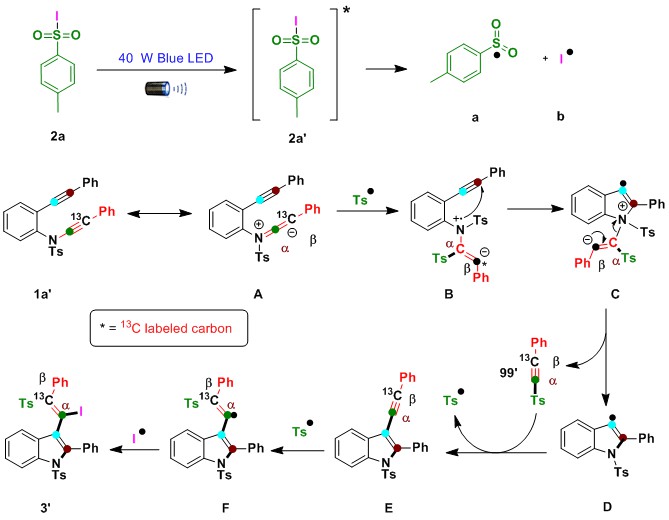
Fig. 3 Plausible reaction mechanism. Blue LED light initiated ynamide C(sp)-N bond cleavage, intramolecular alkyne reshuffling and functionalization.
Based on the control experiment results in this article, previous reports and our own research experience, a plausible mechanism is depicted in Fig. 3 for compound 3’. Blue LED light activate the radical precursor 2a (TsI) to the excited state 2a’. Then homolytic bond fission of excited state 2a’ to generate sulfone (a) and iodine (b) radicals.10 The generated reactive sulfone radical (a) triggers regio- and chemoselectively on the α-carbon of isomerized ynamide intermediate A, producing nitrogen-center radical-cation intermediate B and then inducing selective 5-endo-dig cyclization with an alkyne to generate key reactive intermediate C. The nucleophilic β-carbon of ynamide pushes electrons toward nitrogen cations via C(sp)-N bond fission to generate key intermediate D. Simultaneously, intramolecular migration and insertion of 1-methyl-4-((phenylethynyl-2-13C)sulfonyl)benzene (99’) by discharging sulfone radicals via Ts-C(sp) homolytic bond fission generates another key intermediate E. Finally, liberated radical sulfone addition to the β-carbon of the alkyne produces vinyl radical intermediate F. The available second radical source (iodine radical/selenium) binds with intermediate F to produce the final product 3’.
For more details, especially on product derivatization with various radical precursors and mechanistic studies for the photoinduced radical triggered ynamide C(sp)-N bond fission, migration and functionalization under Blue LED light, please have a look at our article:
https://www.nature.com/articles/s41467-022-30001-7
References
- Hu, Y.-C., Zhao, Y., Wan, B. & Chen, Q.-A. Reactivity of ynamides in catalytic intermolecular annulations. Soc. Rev. 50, 2582-2625 (2021).
- Chen, Y.-B., Qian, P.-C. & Ye, L.-W. Bronsted acid-mediated reactions of ynamides. Soc. Rev. 49, 8897-8909 (2020).
- Mahe, C. & Cariou, K. Ynamides in Free Radical Reactions. Synth. Catal. 362, 4820-4832 (2020).
- Wang, L., Lu, C., Yue, Y. & Feng, C. Visible-Light-Promoted Oxo-Sulfonylation of Ynamides with Sulfonic Acids. Lett. 21, 3514-3517 (2019).
- Tan, T.-D., Wang, Z.-S., Qian, P.-C. & Ye, L.-W. Radical Reactions of Ynamides. Small Methods 5, 2000673 (2021).
- Dutta, S., Mallick, R. K., Prasad, R., Gandon, V. & Sahoo, A. K. Alkyne Versus Ynamide Reactivity: Regioselective Radical Cyclization of Yne-Ynamides. Chem., Int. Ed. 58, 2289-2294 (2019).
- Dutta, S., Prabagar, B., Vanjari, R., Gandon, V. & Sahoo, A. K. An unconventional sulfur-to-selenium-to-carbon radical transfer: chemo-and regioselective cyclization of yne-ynamides. Green Chem. 22, 1113-1118 (2020).
- Zeng, L., Lin, Y., Li, J., Sajiki, H., Xie, H. & Cui, S. Skeletal reorganization divergence of N-sulfonyl ynamides. Commun. 11, 5639 (2020).
- Qi, L.-J., Shi, C.-Y., Chen, P.-F., Li, L., Fang, G., Qian, P.-C., Deng, C., Zhou, J.-M. & Ye, L.-W. Gold-Catalyzed 1,1-Carboalkoxylation of Oxetane-Ynamides via Exocyclic Metal Carbenes: Divergent and Atom-Economical Synthesis of Tricyclic N-Heterocycles. ACS Catal. 11, 3679-3686 (2021).
- H. T., Ito, O., Iino, M. & Matsuda, M., Studies of sulfonyl radicals. 4. Flash photolysis of aromatic sulfones. J. Phys. Chem. 82, 314-19 (1978).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Latest Content
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in