Pick up and dispose of pollutants from water via temperature-responsive micellar copolymers on magnetite nanorobots
Published in Materials

Water contamination is becoming a more serious issue due to fast-growing chemical industries belonging to the pharmaceutical, pesticides and specialty chemicals industries. According to a WHO (World Health Organization) survey, an estimated 485,000 people died last year due to drinking polluted water and a billion cases of various health problems were reported due to waterborne illnesses. Therefore, the removal of toxic contaminants from water has become critical. Water purification by the removal or degradation of contaminants can be done using various techniques such as chemical precipitation, adsorption, membrane filtration, and ion exchange. These techniques are usually time-consuming and have low efficiency due to slow diffusive mass movement. Recently, interest in low-cost and high-efficiency technology development for water purification has prompted the establishment of nanotechnology-based cutting-edge approaches. Environmental remediation has prompted interest in “self-propelled nano/micromotors” that are capable of autonomous movement while utilizing external stimuli or local fuels. Compared with static remediation nanomaterials, nano/micromotors have superior adsorption ability and faster treatment times due to faster mass transport and efficient fluid mixing.
Recently, many types of remediation materials have been used in the fabrication of nano/micromotors due to their outstanding structural and surface characteristics. The magnetically driven nano/micromotors are an instance of newly developed artificial motors for efficient water cleaning and environmental remediation. However, the majority of magnetic nanomotors utilized in water purification depend on costly metal catalysts (e.g., gold and platinum) and a complex synthesis process, which generates plenty of issues in scale-up and real-world applications.
In the present work, a cost-effective and unique method for water decontamination has been demonstrated using temperature-responsive magnetic nanorobots (TM nanorobots). The TM nanorobots have been developed by integrating pluronic block-copolymers with magnetite (Fe3O4) nanoparticles. The Fe3O4 nanoparticles serve as an active material for magnetic propulsion while the pluronic copolymer works as molecular “hands” to remove pollutants. The components utilized in TM nanorobots are superior to other synthetic adsorbent materials as they are less expensive, biodegradable, and most importantly, less hazardous. Figure 1 shows the TM nanorobots pickup and disposal of toxic pollutants from the water via inter-micellar agglomeration and separation of the copolymer at different temperatures.
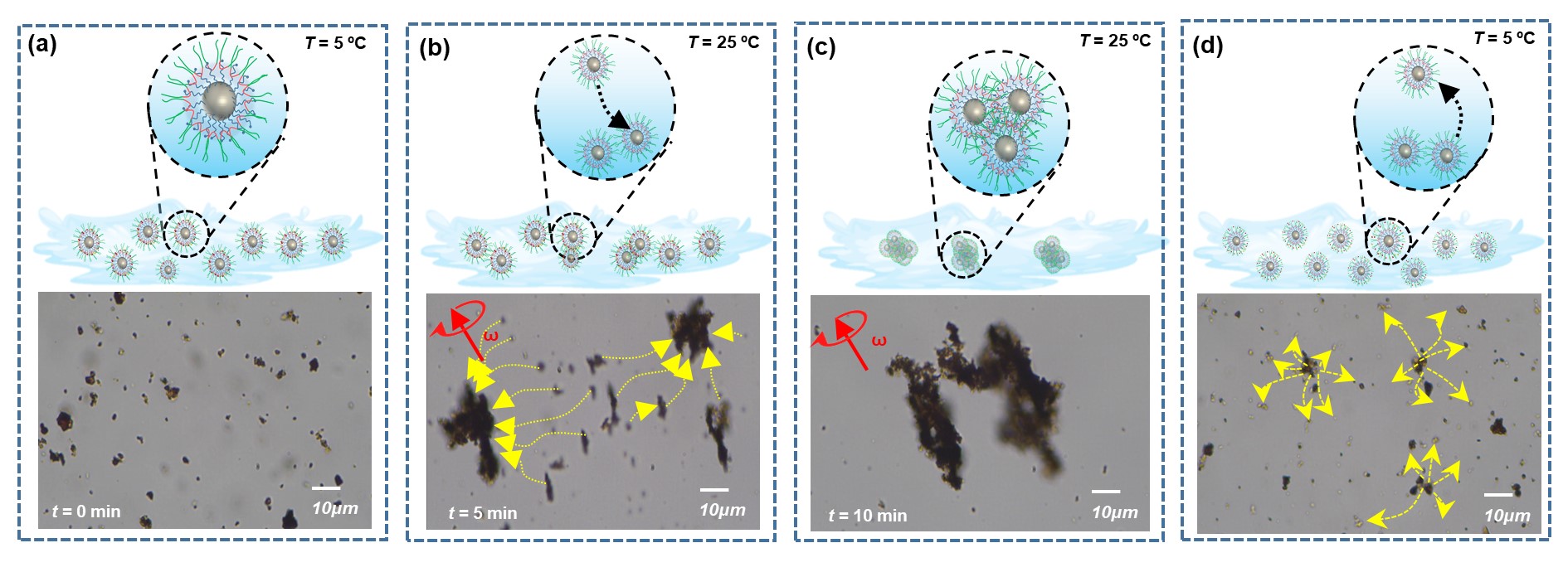
The inherent characteristic of pluronic copolymers is their thermo-responsive micellization in aqueous media over a wide temperature range based on the molecular weight and poly(propylene oxide) (PPO)/(poly(ethylene oxide) (PEO) composition. The copolymer chains are fully extended due to hydration at critical micellization temperature (CMT, ~5 oC), which allows pollutants to enter into the polymeric matrix with the water. When the temperature rises over the CMT (~25 °C), the copolymer dehydrates and stronger interactions between the polymer blocks (PEO/PPO) can trap the pollutants. Simultaneously, a transversal rotating magnetic field supplied to the TM nanorobots aids in the interaction of polymer blocks on the nanorobots and allows for effective aggregation. Following that, the subsequent cooling reopens the polymeric chain and segregates the TM nanorobots, allowing the pollutant to be disposed. Due to the magnetic sensitivity provided by Fe3O4, an external magnetic field could be used to direct the TM nanorobots to the desired location.
The maximum pickup efficiency of toxic metal (arsenic) and pesticide (atrazine) by the TM nanorobots was 65.2% and 61.5%, respectively, whereas the disposal process reached equilibrium after 100 min, with arsenic efficiency of 48% and atrazine efficiency of 38.4%. Notably, the dynamic pickup efficiency of both pollutants on TM nanorobots is enhanced two-fold over the static system. In addition, TM nanorobots demonstrated outstanding pickup efficiency of pollutants in real contaminated complex water samples (e.g., real tap and river water). The introduction of high molecular weight or graft pluronic copolymers in the nano/micromotors could further improve the pickup capacity by enhancing functional groups and adsorption sites. This research could pave the way for the development of various surface-functionalized magnetite nanorobots to address new environmental remediation requirements.
For further information, please read our published article in Nature Communications, https://doi.org/10.1038/s41467-022-28406-5.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in