Polymicrobial Infections – A Life-Long Tug of War between Immunity and Pathogens
Published in Microbiology, Plant Science, and General & Internal Medicine

A bacterial – fungal tug of war in mixed biofilms
Several reports demonstrate that microbial pathogens, including Pseudomonas aeruginosa, Candida albicans, Staphylococcus aureus, Aspergillus fumigatus, Cryptococcus neoformans and Klebsiella pneumoniae engage in interspecies interactions that could impact the outcome of infectious diseases.1-3 In this work4, we took efforts towards a better understanding of the interaction of two highly relevant pathogens, the environmental fungus Aspergillus fumigatus (Afu) and the bacterial commensal Klebsiella pneumoniae (Kpn). We employed omics technologies such as genomics, proteomics and metabolomics to generate integrated correlative datasets to characterize the dynamic interaction of two clinically relevant pathogens that share the respiratory tract as the major niche in humans. We show that Kpn encountering Afu in mixed biofilms drives for fungal (Afu) adaptation through altered energy metabolism and secondary metabolite production. Although Kpn exerts detrimental effects, a remarkable metabolic plasticity enhances Afu fitness, allowing for a co-existence of the fungus with Kpn in mixed biofilms. Importantly, the data identify potential biomarkers that could facilitate clinical interventions to mitigate detrimental effects of mixed biofilms. The work also specifies transcription factor networks that control hallmark responses in Afu, including secondary metabolite pathways that are activated by interspecies interactions.
While the work represents a first pivotal step towards a better understanding of polymicrobial infections between these specific pathogen partners, the results do raise additional important questions about the in vivo behaviour of mixed biofilms. For example, could the outgrowth of K. pneumoniae upon superinfections with A. fumigatus create a permissive environment for long-term co-colonization by A. fumigatus in patients? This could pose a serious risk, since Afu is a strong trigger for chronic allergies as well as pulmonary aspergillosis5, 6 . Further, how does the host immune defense cope with superinfections that could emerge from mixed biofilms in vivo? Is either microorganism triggering a pathogen-specific inflammatory response following immune activation? In other words, Kpn is a commensal normally tolerated by immune surveillance, but could Afu also activate the immune defense against Kpn to restrain bacterial outgrowth? How do host defense pathways cooperate to clear mixed polymicrobial infections without causing overshooting inflammatory responses that usually drive fatal septic outcomes?
As we realized, studying polymicrobial infections is challenging. There are serious limitations due to potential confounding effects that impact experimental design as well as data quality. First, culture conditions and growth media in vitro differ greatly from the situation Afu and Kpn encounter in respiratory tissues in vivo. Second, although both microbes in mixed biofilms establish a homeostatic relationship enabling their co-existence, the pathogens exert reciprocal attack and defense strategies, leading to a fitness equilibrium both in vitro and in vivo. However, both microbes striving in biofilms in vivo will also encounter immune defense, which most likely affects their fitness in distinctive ways. Hence, pathogen-specific adaptive responses to immunity are likely to affect polymicrobial interactions in vivo. In other words, host defense may impose additional dynamics and complexity concerning virulence of individual pathogens in mixed biofilms. Of note, immune surveillance tolerates the presence of a commensal such as Kpn up to certain levels, but generally aims at removing true pathogens such as Afu. Unfortunately, animal models facilitating suitable in vivo studies of polymicrobial infections are either unavailable or suboptimal. Thus, there is an unmet need for more adequate animal models to conduct in vivo studies on mixed infections. Deciphering the molecular dynamics of superinfections involving multiple pathogens remains challenging.
Looking ahead - how can we better cope with the burden of polymicrobial infections?
Polymicrobial infections pose enormous clinical challenges and raise many critical questions 7. How can we better understand the complexity of onset and progression, and how will it be possible to better identify causalities, especially in the context of concomitant microbiome dysbiosis? How can we better tackle superinfections, improve fast and species-specific clinical diagnostics to improve current treatment regimens? To answer some of these questions, we must address the urgent need for a deeper understanding of interspecies metabolic activities and exchange, as well as beneficial and detrimental microbiome interactions in the context of diminishing host defense. Further, host-, pathogen- as well as disease-specific signatures are urgently needed, because they may yield better correlations as to how microbiome dynamics impact infections. This started to be addressed through phenotypic profiling data of single microbiome constituents residing in host tissue microenvironments8. It is equally critical to understand the onset and spread of antimicrobial resistance (AMR) within microbial populations, as well as their correlation with inadequate or suboptimal antibiotic treatment 9. We strongly believe that elucidating microbiome dynamics in the response to exogenous (environmental) as well as endogenous commensal triggers of dysbiosis are pivotal steps towards a better understanding of pathogen-specific causalities in infectious diseases. Unfortunately, many current challenges seem unsurmountable owing to a “grand canyon” separating and hampering the translation of in vitro or in vivo data from animal models to actual outcome in patients.
Next generation sequencing technologies have been instrumental for “stamp-collecting” omics efforts, since they permitted identifying organismal and genetic blueprints for any microbiome 10. However, the real challenges lie ahead when it comes to unraveling genotype-phenotype correlations in vivo, including pathogen-specific plasticities facilitating immune evasion. Even when considering all available technologies, the unimaginable complexities arising from genetic interactions as they orchestrate microbial metabolism, fitness, growth or dysbiosis constitute serious roadblocks to experimental approaches.
Strikingly, artificial intelligence (AI) approaches will most likely be THE game changers, given the lightning pace by which AI applications that affect all aspects of life are emerging on a daily basis, including medical and healthcare implications 11, 12. AI approaches will be the glue for integrating and understanding large-scale patient datasets, which could be further facilitated due to the availability of anonymized but disease-specific healthcare datasets accessible in global databases. AI has already revolutionized protein structure analysis by accurately predicting de novo protein folding, which is one of the most dynamic and complex molecular processes in living cells13. Thus, it is reasonable to predict that avalanches of emerging AI applications will allow for elucidating and predicting the onset, progression, treatment and outcome of pathogenic infections. AI will be a foundation for personalized medicine towards improving the well-being of at-risk individuals long before the onset of diseases. By integrating genomics, proteomics, metabolomics, as well as phenomics datasets, AI tools and machine learning will model and predict microbial interactions, map mutual and reciprocal exchanges of metabolites within microbiomes and with the host, and determine how these factors orchestrate the dynamics of microbial interplay in host niches. Furthermore, AI will be beneficial to uncover underlying mechanisms, thus revealing better treatment options for polymicrobial infections through precision medicine strategies. Finally, AI will certainly help in relieving economic restraints many healthcare systems are currently suffering from owing to high costs of preventive, and short- or long-term treatment strategies 14. For example, AI-driven tools can support the prediction of both inherent and acquired anti-infective drug resistance patterns based on individual microbiome or pathogen data 15, further paving the way for cost-effective personalized therapies. Taken together, AI holds an as yet untapped potential to yield a better understanding of medical conditions ranging from cancer to infectious disease or even autoimmunity. Moreover, AI is expected to propel translational medicine, bridging the gap from bench to bedside, including the prediction of infection trajectories and outcomes.
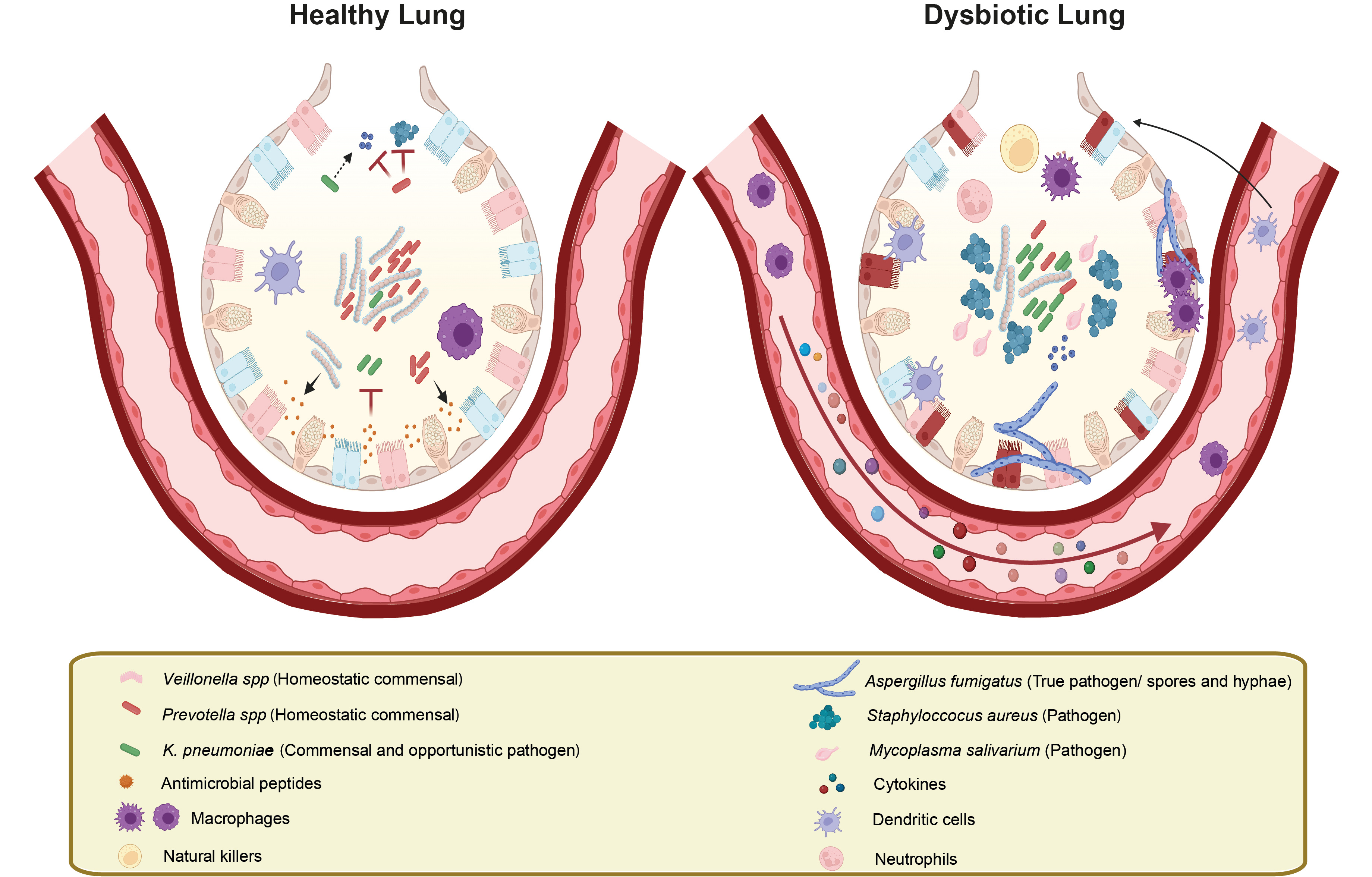
Lung barrier microenvironment in health and disease. In a healthy lung barrier, the microbiome (left) is subject to immune surveillance engaging both innate and adaptive immune cells. Immune defence relies on the secretion of antimicrobial peptides and signalling molecules that allow for keeping the pathogenic potential of commensal colonizers like Klebsiella pneumoniae in check. The physiological lung barrier is home to hundreds of microbial colonizers, including beneficial microbes such as Prevotella spp and Veillonella spp that limit colonization of commensals like Staphylococcus aureus16 or the environmental fungal pathogen Aspergillus fumigatus. By contrast, dysbiosis triggers (right) disruption of the balanced equilibrium in the homeostatic lung microbiome, facilitating the overgrowth of certain pathobionts that can evade the host immune defense. The imbalance also causes immune overactivation yet it allows for an altered microbial community with increased pathogenic potential. Elevated levels of inflammatory and chemotactic mediators released by the host activate resident as well as recruited innate and adaptive immune cells, all in all promoting hyperinflammatory conditions. Image created with BioRender and Illustrator.
Contributors: Tamires Bitencourt 1,2,3, Thomas Lion2,3,4 & Karl Kuchler1*
From the
1Medical University of Vienna, Max Perutz Labs Vienna, Department of Medical Biochemistry
2CCRI – St. Anna Children's Cancer Research Institute, Vienna, Austria,
3Labdia - Labordiagnostik GmbH, Vienna, Austria,
4Department of Pediatrics, Medical University of Vienna, Austria.
*Corresponding author
Karl Kuchler
E-mail: kuchlerkarl1@univie.ac.at
Acknowledgements
We apologize to all authors whose contributions we were unable to cite owing to space restrictions.
References
- Margalit, A., Carolan, J.C. & Kavanagh, K. Bacterial Interactions with Aspergillus fumigatus in the Immunocompromised Lung. Microorganisms 9 (2021).
- Allison, D.L. et al. Candida-Bacteria Interactions: Their Impact on Human Disease. Microbiol Spectr 4 (2016).
- Sukumaran, A., Ball, B., Krieger, J.R. & Geddes-McAlister, J. Cross-Kingdom Infection of Macrophages Reveals Pathogen- and Immune-Specific Global Reprogramming and Adaptation. mBio 13, e0168722 (2022).
- Bitencourt, T. et al. Integrated multi-omics identifies pathways governing interspecies interaction between A. fumigatus and K. pneumoniae. Commun Biol 7, 1496 (2024).
- Sengupta, A. et al. Mortality in chronic pulmonary aspergillosis: a systematic review and individual patient data meta-analysis. The Lancet Infectious Diseases (2024).
- Sehgal, I.S. et al. Is There an Overlap in Immune Response Between Allergic Bronchopulmonary and Chronic Pulmonary Aspergillosis? J Allergy Clin Immunol Pract 7, 969-974 (2019).
- Short, F.L., Murdoch, S.L. & Ryan, R.P. Polybacterial human disease: the ills of social networking. Trends Microbiol 22, 508-516 (2014).
- Wu, G. et al. A core microbiome signature as an indicator of health. Cell (2024).
- Ghuneim, L.J. et al. Complex and unexpected outcomes of antibiotic therapy against a polymicrobial infection. ISME J 16, 2065-2075 (2022).
- Hou, K. et al. Microbiota in health and diseases. Signal Transduct Target Ther 7, 135 (2022).
- Koch, M. Artificial Intelligence Is Becoming Natural. Cell 173, 531 (2018).
- Dave, M. & Patel, N. Artificial intelligence in healthcare and education. Br Dent J 234, 761-764 (2023).
- Abriata, L.A. The Nobel Prize in Chemistry: past, present, and future of AI in biology. Commun Biol 7, 1409 (2024).
- Silcox, C. et al. The potential for artificial intelligence to transform healthcare: perspectives from international health leaders. NPJ Digit Med 7, 88 (2024).
- Ho, C.S. et al. Antimicrobial resistance: a concise update. The Lancet Microbe (2024).
- Natalini, J.G., Singh, S. & Segal, L.N. The dynamic lung microbiome in health and disease. Nat Rev Microbiol 21, 222-235 (2023).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in