Polyvanadate in metal-organic framework boosting peroxymonosulfate activation
Published in Ecology & Evolution

As present, lots of regions were facing the water environmental issues induced by emerging contaminants, which would cause series of problems like the generation of super bacteria, the disturbance of organism's endocrine system and so on. Thus, it was necessary to exploit effective treatment technologies to reduce the persecution of organics on human health and the ecological environment. In various wastewater treatment technologies, Fenton-like reaction triggered by persulfates (peroxydisulfate (PDS) and peroxymonosulfate (PMS)) activation had been verified to be promising technology to achieve organics elimination, profiting from the production of radicals and/or non-radicals via interacting between persulfates and active centers. One of the significant points of Fenton-like technology was the exploitation of catalysts with high active sites. Our research group from the Beijing University of Civil Engineering and Architecture, Peking University Shenzhen Graduate School and East China University of Science and Technology devoted to developing the environmental functional materials like metal-organic frameworks (MOFs), metal-based catalysts and carbon materials as well as the applications in water environment remediation.
High active centers in catalysts could efficiently accomplish catalytic persulfates activation via electron transfer process to produce various reactive oxygen species. Thus, many previous studies were devoted to pursuing higher electron transfer efficiency during the Fenton-like reactions, which could produce abundant active species for organics elimination. Different from the single atom-based catalysts, most of MOFs based catalysts possessed complicated surface coordination environment, which could result in the multiple binding formations between PDS/PMS and the catalyst surface. Multiple binding formations usually might induce multi-channel electron transfer process, also signifying that the corresponding persulfates excitation process was complicated. Therefore, we thought that PS activation processes don’t solely rely on electron transfer from dominant metal centers. And it was of great significance to explore the catalytic activation mechanism of persulfates in Fenton-like reaction for the subsequent direct regulation of catalyst structure and the investigation of structure-activity relationship.
To verify the above discussion, we constructed a polyoxovanadate-based Co2(V4O12)(bpy)2 to achieve PMS activation for organic pollutants degradation in this study, deeply investigating the relationship between the complex surface coordination environment of catalyst and Fenton-like reaction. The Co2(V4O12)(bpy)2 displayed the satisfactory PMS activation for various electron-rich pollutants degradation with high reaction apparent rate constant, in which the reaction apparent rate constant of Co2(V4O12)(bpy)2 surpassed almost all reported catalysts for PMS activation. Subsequently, this study confirmed that catalytic PMS activation processes don't solely rely on electron transfer from Co centers due to the complicated composition and interface environment of catalysts. In-situ characterizations and density functional theory (DFT) calculations affirmed that the terminal oxygen in [V4O12]4− could interact with the terminal hydrogen of PMS to form the hydrogen bond. And the electrons stored in the ‘electron sponge’ could be directly transferred to the O-H bond of PMS through hydrogen bonding due to the ‘electron sponge’ effect of [V4O12]4−, further achieving the formation of SO5•− intermediate. Subsequently, generated singlet oxygen and surface-bound sulfate radical by SO5•− intermediate co-degraded series of electron-rich micropollutants from water, which not only displayed excellent environmental anti-interference ability, but also realized the good removal efficiency of total organic carbon. The continuous operation device proved that Co2(V4O12)(bpy)2 possessed good practical application prospect, in which the volume of wastewater treated per gram could reach 120.0 L. In all, this work not only presented an efficient catalyst with electron sponge for water environmental remediation via nonradical pathway, but also provided the fundamental insights into the Fenton-like reaction mechanism.
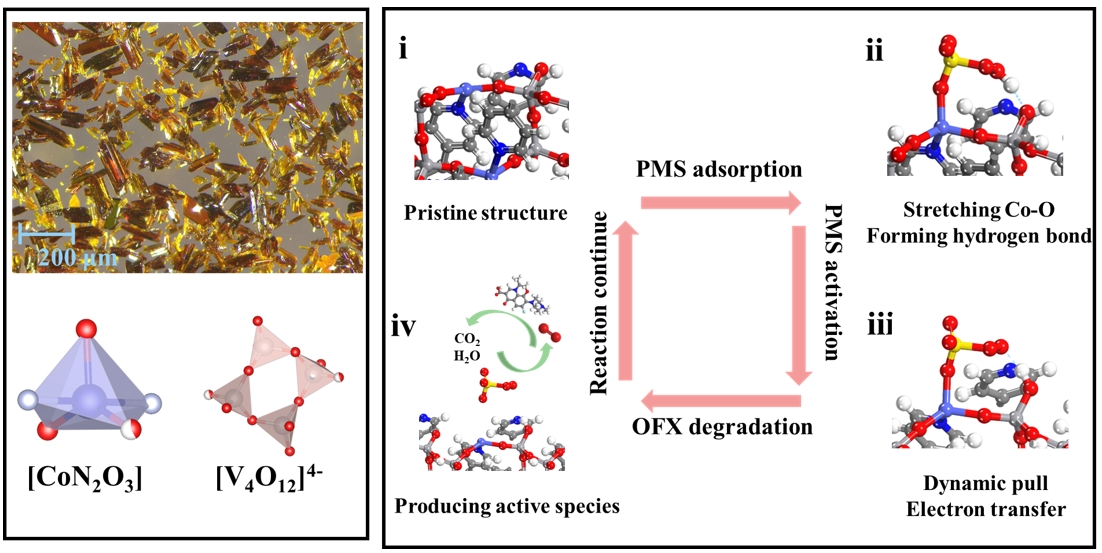
Figure: The optical microscope image of Co2(V4O12)(bpy)2 and coordination modes of Co, V (left). Stepwise analyses of PMS activation mechanism during Fenton-like reaction (right). Image author: Prof. Dr. Chong-Chen Wang
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in