Population pharmacokinetic modelling of primaquine exposures in lactating women and breastfed infants
Published in Pharmacy & Pharmacology
Motivation and Background
Malaria remains a significant global health concern, with millions of cases reported annually. Primaquine is a crucial drug in the fight against malaria due to its effectiveness in targeting the dormant liver stage (hypnozoite) of vivax and ovale malaria, thereby preventing relapse and contributing to disease eradication. Additionally, primaquine is used to block the transmission of malaria by killing gametocytes, the stage of the parasite that is transmitted to a mosquito during a blood meal.
Primaquine is a safe and effective drug, but it can cause severe side effects (haemolysis) in patients with a specific genetic mutation (glucose-6-phosphate dehydrogenase (G6PD) deficiencies). A study at the Thailand-Myanmar border evaluated the tolerability and safety of a single low dose of primaquine (0.25 mg/kg) in 819 patients and demonstrated that this dose was well tolerated, did not cause clinically significant haemolysis in G6PD-deficient individuals, and can be used safely without prior G6PD testing.
Limited detailed pharmacokinetic information on primaquine use in breastfeeding mothers and its excretion into breast milk restricts its utilization. Guidelines advise against primaquine treatment in breastfeeding mothers due to the perceived risk of haemolysis in G6PD-deficient infants. These guideline restrictions on both pregnant and breastfeeding mothers exclude approximately 13% of women from receiving radical cure treatment for malaria. Removing the restriction on breastfeeding mothers could potentially reduce this to only 4%.
Our study addressed this crucial gap by investigating the pharmacokinetic properties of primaquine in breastfeeding mothers and its transfer into breast milk. We predicted the amount of drug received from the breast milk and the resulting infant drug exposure to assess the risk of haemolytic side effects. This knowledge could potentially expand the use of primaquine radical cure to include breastfeeding mothers.
Study Design and Methodology
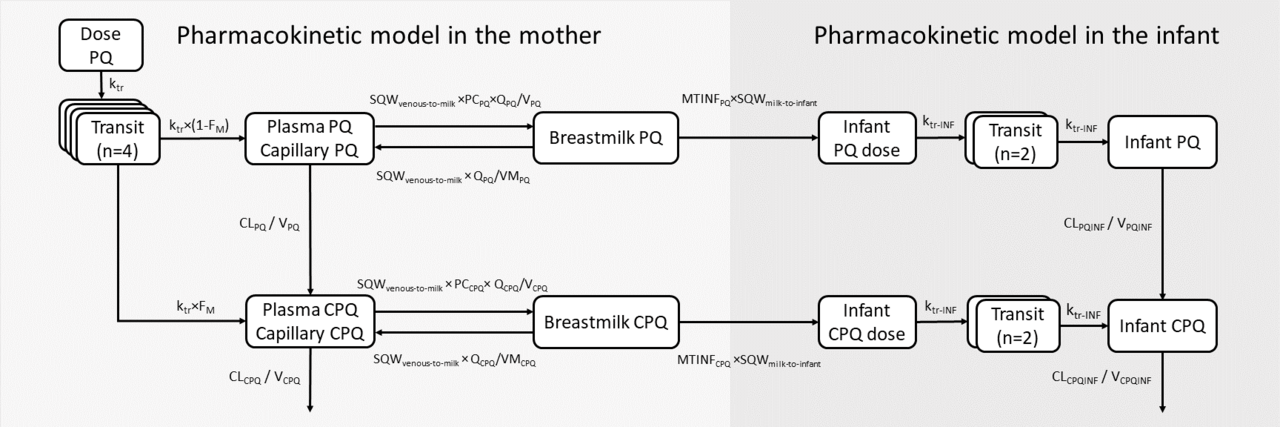
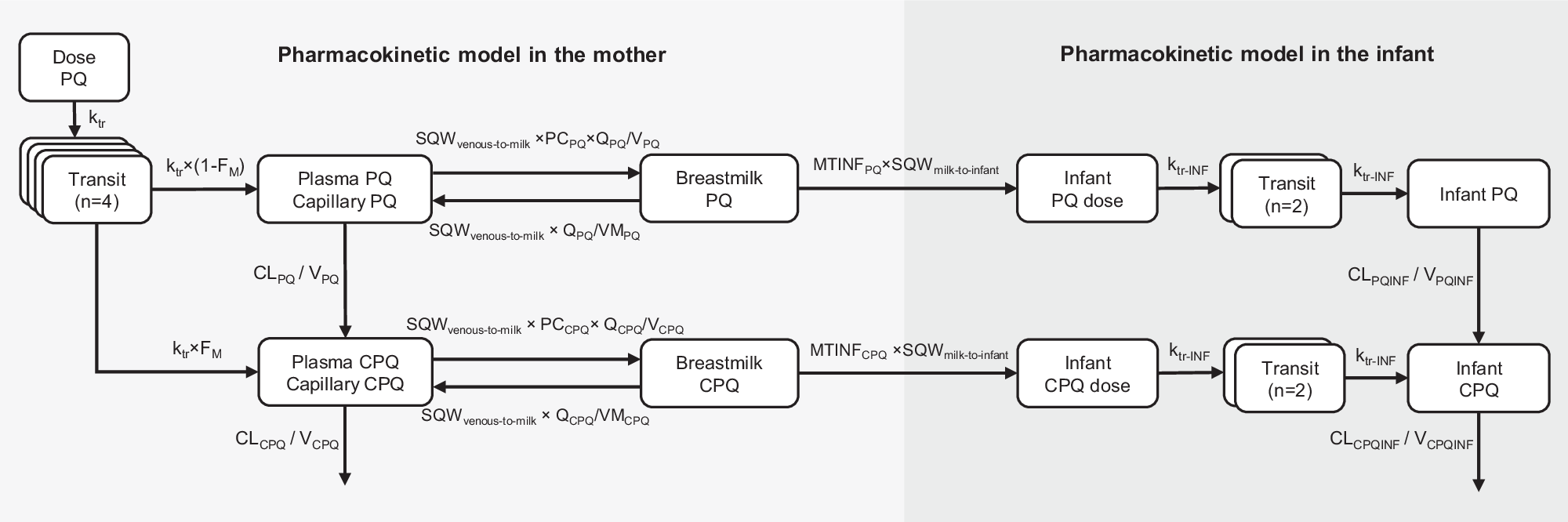
To characterize the pharmacokinetic properties of primaquine in breastfeeding mothers and to predict infant exposure through breast milk, we utilized data from a published study involving twenty-one G6PD-normal breastfeeding mothers and their G6PD-normal infants aged between 28 days and 2 years. The mothers were administered the standard dose of primaquine for 14 days (0.5 mg/kg/day). Serial pharmacokinetic samples from plasma and breast milk were collected from the mothers, and capillary samples were obtained from the infants. With this data, we employed nonlinear mixed-effects modelling to develop a population pharmacokinetic model for the mothers, estimate the fraction of primaquine excreted into the breast milk, and calculate the relative infant dose. Furthermore, a mother-to-infant model was developed to mimic the infant feeding pattern and predict infant drug exposure after administering various dosage regimens of primaquine to the mothers (0.25-1 mg/kg once daily for 1-14 days).
Key Findings
Primaquine transfer through breast milk:
Our results showed that primaquine can be distributed from the mothers' plasma into the breast milk, resulting in approximately 40% of the concentrations seen in plasma. However, the relative infant dose, which represents the amount of drug an infant received through breast milk, was approximately 0.5% of the dose that the mother received. The systemic concentrations observed in breastfed infants were significantly lower than those seen in their mothers, with only one sample of primaquine being measurable in 1 out of 21 infants. This suggests that while the drug does reach the infants, the exposure is very limited, which suggests a minimal potential for haemolytic effects in the infants.
Exposure in breastfed infants:
The results from the developed mother-to-infant model showed that total primaquine exposures in infants were less than 1% of the exposure seen in mothers. Additionally, predicted exposure in infants in all dosing scenarios was well below the exposure in mothers receiving a single low dose of primaquine (0.25 mg/kg), a dose known to be safe and not cause haemolysis in individuals with G6PD deficiencies. Therefore, even in infants with the most severe G6PD deficiency variants, it is highly unlikely that giving standard doses of primaquine to the mothers would cause haemolysis in breastfed infants.
Safety profile:
None of the infants in the study experienced haemolysis, which is an encouraging finding. It supports the safety of administering primaquine to breastfeeding mothers without posing significant risks to their breastfed infants. This data can reassure healthcare providers and patients about the safe use of primaquine during lactation.
Implications for clinical practice
The implications of our findings are significant for clinical practice. The data supports the safe use of primaquine in lactating women, which is crucial for effective malaria treatment and prevention. This is particularly important in regions where malaria is endemic and the risk of relapse due to P. vivax is high. By ensuring that lactating women can be safely treated with primaquine, we can better protect both mothers and their infants from the devastating effects of malaria.
Additionally, the pharmacokinetic data generated from this study can inform guidelines and recommendations for healthcare providers. With a clearer understanding of primaquine’s behaviour in lactating women, clinicians can make more informed decisions regarding dosing and monitoring, ultimately improving patient care and outcomes.
Future directions and conclusions
While our study provides valuable insights, expanding the research to include a larger and more diverse population will help to validate our findings and enhance their generalizability. Furthermore, the breast milk data collected in the current study was from mature milk, which has smaller amounts of fat and a lower pH value compared to colostrum. The impact of different breast milk compositions in the early breastfeeding period is currently being assessed in an ongoing clinical study (ClinicalTrials.gov Identifier: NCT04984759). This study will examine primaquine concentrations in colostrum, and a revised pharmacokinetic model for evaluating drug exposure in these women could be developed when the data become available.
In conclusion, the results from this rare study in a vulnerable group of patients contribute significantly to our understanding of the pharmacokinetics of primaquine in breastfeeding mothers and its excretion to breast milk. The results suggest very low infant exposures to primaquine compared to maternal exposures. Such exposures are very unlikely to pose any haemolytic risk, even in the most severe variants of G6PD deficiency. This supports the use of primaquine for radical cure during breastfeeding after the neonatal period.
References
World Health Organization. World malaria report 2023. Geneva, Switzerland: WHO Press; 2023. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023.
World Health Organization. Guidelines for the treatment of malaria. Third edition. Geneva, Switzerland: WHO Press; 2015. Available from: https://www.afro.who.int/publications/guidelines-treatment-malaria-third-edition.
Bancone G, Chowwiwat N, Somsakchaicharoen R, Poodpanya L, Moo PK, Gornsawun G, Kajeechiwa L, Thwin MM, Rakthinthong S, Nosten S, Thinraow S, Nyo SN, Ling CL, Wiladphaingern J, Kiricharoen NL, Moore KA, White NJ, Nosten F. Single Low Dose Primaquine (0.25 mg/kg) Does Not Cause Clinically Significant Haemolysis in G6PD Deficient Subjects. PloS one. 2016;11(3):e0151898.
Watson J, Taylor WRJ, Bancone G, Chu CS, Jittamala P, White NJ. Implications of current therapeutic restrictions for primaquine and tafenoquine in the radical cure of vivax malaria. PLoS neglected tropical diseases. 2018;12(4):e0006440.
Gilder ME, Hanpithakphong W, Hoglund RM, Tarning J, Win HH, Hilda N, Chu CS, Bancone G, Carrara VI, Singhasivanon P, White NJ, Nosten F, McGready R. Primaquine Pharmacokinetics in Lactating Women and Breastfed Infant Exposures. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2018;67(7):1000-7.
Wattanakul T, Gilder ME, McGready R, Hanpithakpong W, Day NPJ, White NJ, Nosten F, Tarning J, Hoglund RM. Population pharmacokinetic modelling of primaquine exposures in lactating women and breastfed infants. Nature communications. 2024;15(1):3851.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in