Preparation of near-infrared AIEgen-active fluorescent probes for mapping amyloid-β plaques in brain tissues and living mice
Published in Chemistry

Most neurodegenerative conditions are associated with pathological accumulation of one or more folded or misfolded aggregated proteins. Among them, Alzheimer’s disease (AD) has been regarded as an incurable condition, which is the most common cause of dementia1. It is well-known that the accumulation and deposition of amyloid-β (Aβ) peptides into amyloid plaques is considered a pathological biomarker in AD2,3. Therefore, the discovery of approaches for directly mapping of Aβ plaques in vivo would be critical for the early diagnosis of the AD status.
Commercial Thioflavin derivatives (ThT or ThS) have been known as gold standard probes for in vitro histological amyloid assay for about half a century. Despite their widespread usage, the inherent defects including distorted signals from enrichment concentration quenching, low signal-to-noise (S/N) ratio with background interference (from unbound probes and their short emissive wavelength), and difficulty in penetrating the blood-brain barrier (BBB), thereby making these probes unable to in vivo track Aβ plaques in living animals (Fig. 1a). Recently, we have made a major advance in directly in vivo mapping of Aβ plaques (Nat. Protoc. 2023, 10.1038/s41596-022-00789-1).
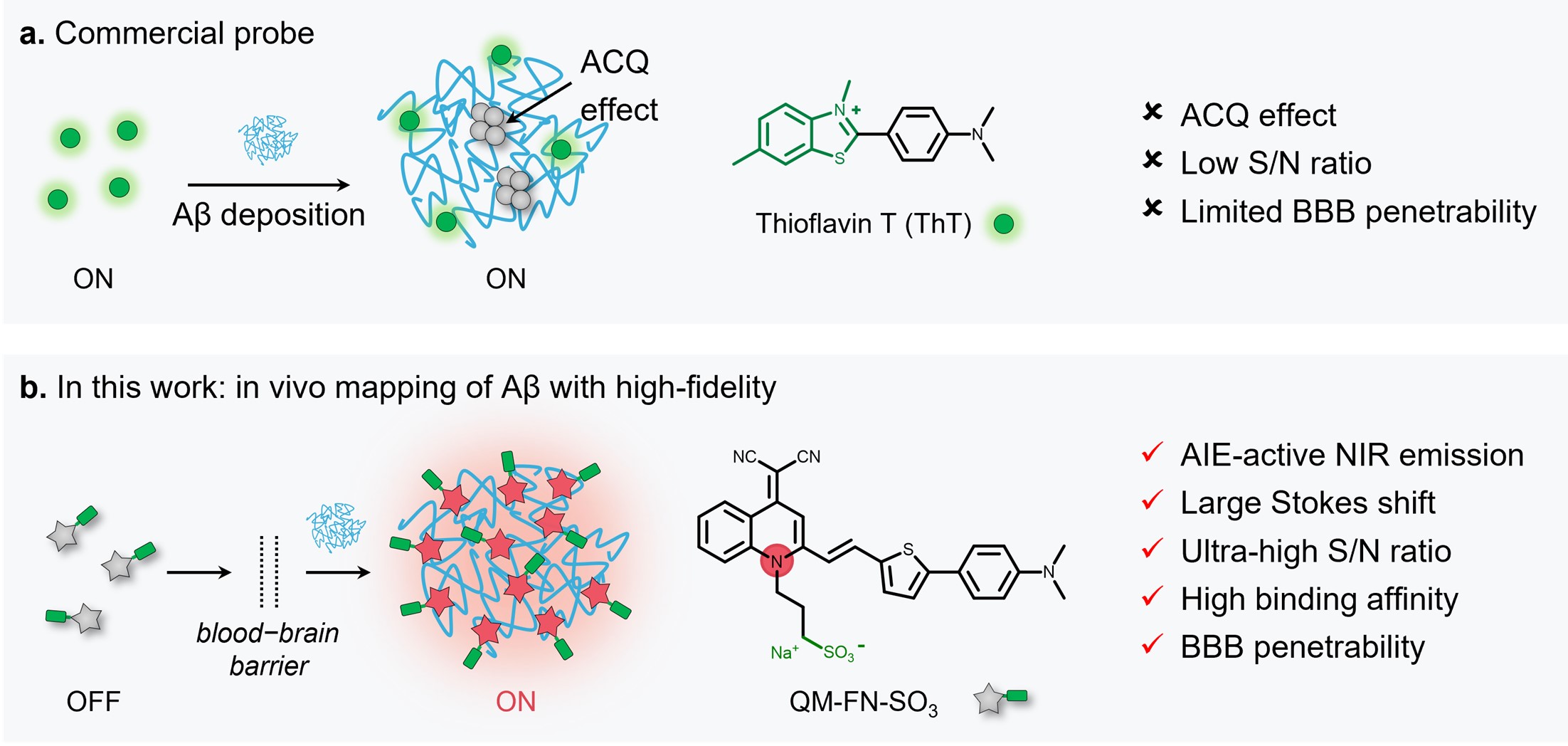
Fig. 1 | Comparison of QM-FN-SO3 with extensively used ThT for sensing Aβ aggregates. a Commercial fluorescent probe ThT (the gold standard for detection of Aβ aggregates): unable to sense Aβ plaques in living animals (ACQ effect, low S/N ratio, and limited BBB penetrability). b Probe QM-FN-SO3 from this protocol: near-infrared aggregation-induced-emission (AIE)-active probe enables in vivo and in situ ultra-sensitively lighting-up Aβ plaques.
In this work, we focus on a “step-by-step” rational design strategy to circumvent the inherent limitation of commercial probes ThT and ThS (Fig. 2a), and thereby make a breakthrough in high-fidelity feedback on in vivo detection of Aβ plaques. Specifically, QM-FN-SO3 possesses unique advantages including a very low background (merely 1/28 times of ThT), ultra-high S/N ratio (8.3-fold than ThT, Fig. 2b), remarkable binding affinity, and excellent photostability (120-fold than FDA-approved near-infrared contrast agent ICG). Furthermore, in vitro experiment results verifies that QM-FN-SO3 could point out and amplify the fidelity signals because of emitting stronger during concentration enrichment with Aβ deposition (Fig. 3a). Finally, in vivo and ex vivo experiments provide solid evidence on the high-fidelity mapping of Aβ plaques in living mice, which is confirmed by colocalization with anti-Aβ antibody-2454 (Fig. 3b and 3c).4,5
By using the probe QM-FN-SO3, all data acquisition and analyses for in vivo lighting-up Aβ plaques in AD animals can be completed within 1 h, and require only a basic knowledge of spectroscopy and chemistry. It is noteworthy that QM-FN-SO3 is now commercially available as a near-infrared (NIR) BioTracker for AD in J&K Scientific Co. Ltd (https://www.jkchemical.com/product/2959021).
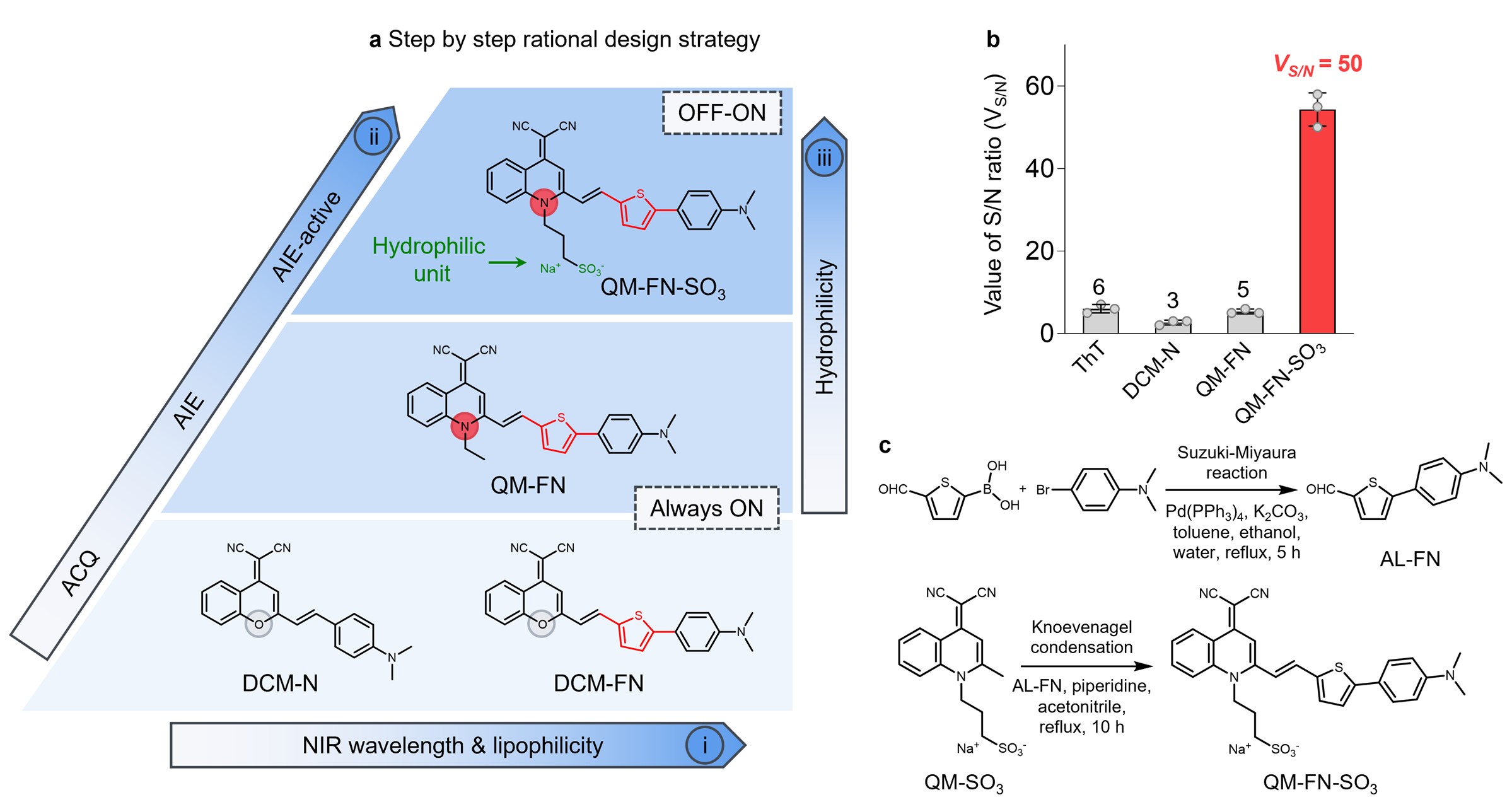
Fig. 2 | Step-by-step strategy for building up probes enabling ultra-sensitive in vivo mapping of Aβ plaques. a The step-by-step strategy: (i) introducing an additional thiophene bridge for extending emission wavelength and matching the lipophilicity requirement for BBB penetrability; (ii) replacing the aggregation-caused quenching (ACQ) building block to our AIEgen quinoline-malononitrile (QM) building block; (iii) attaching with hydrophilic sulfonate unit (marked in green) that achieves a fluorescence-off state in the unbound form. b Value of signal/noise ratio (VS/N) of the probes. c Synthetic route of QM-FN-SO3.
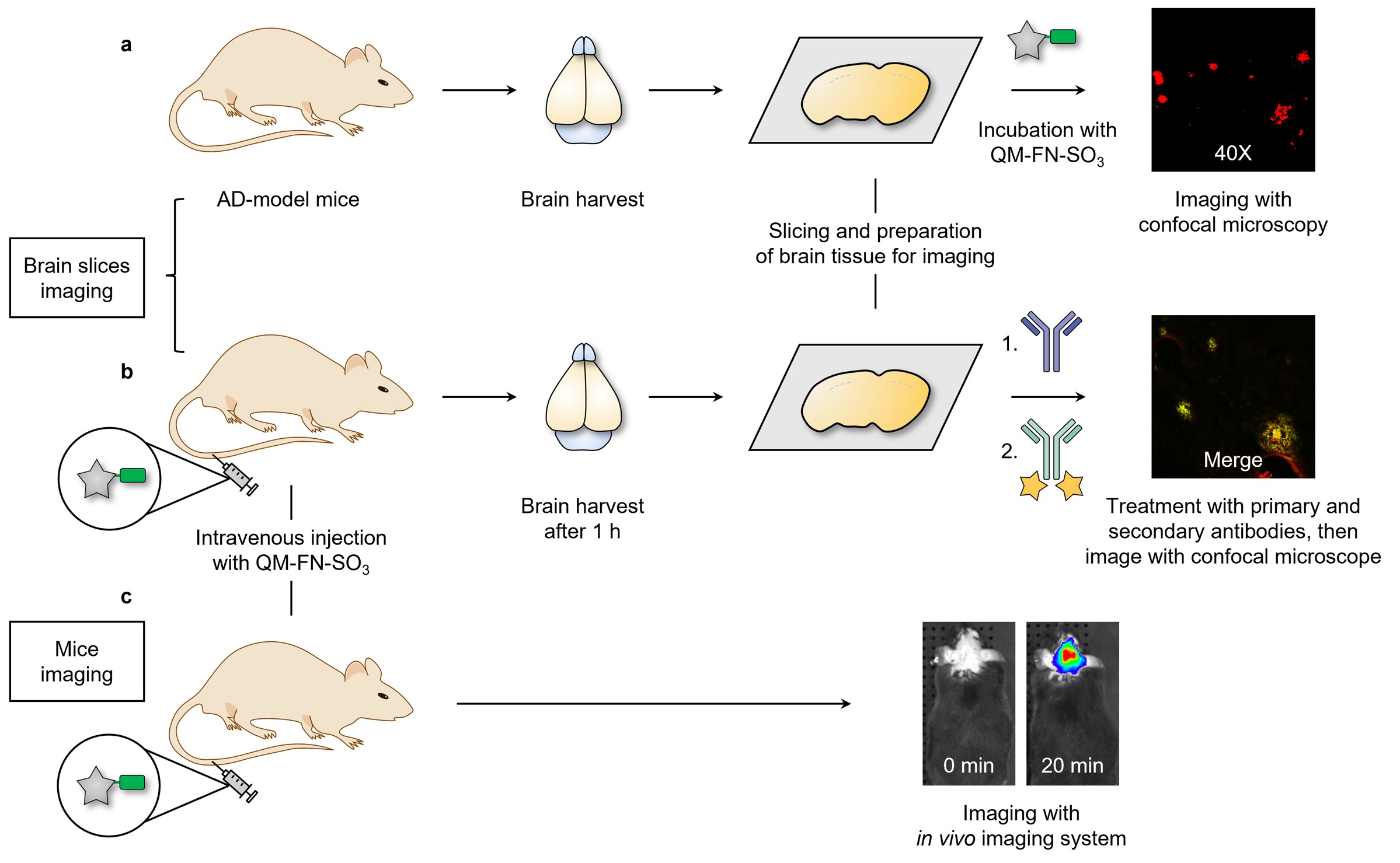
Fig. 3 | Overview of applying QM-FN-SO3 for brain section and animal experiments. a High-fidelity mapping Aβ plaques in brain tissues. b Validation of the BBB penetrability of QM-FN-SO3. c Lighting-up Aβ plaques in living mice.
In summary, we have made a breakthrough in high-fidelity mapping of Aβ plaques, overcoming the dilemma between lipophilic requirement for longer emission and aggregation behavior from water to protein fibrillogenesis. Probe QM-FN-SO3 exhibited extraordinary features of ultra-high S/N ratio, remarkable binding affinity with BBB penetrability, and high-performance near-infrared emission. This work proposes an effective strategy for the design of near-infrared AIE-active probes, serving as a promising alternative to commercial probes. The detection characteristics of QM-FN-SO3 greatly streamline and improve the protocols for Aβ analysis in living animals. We anticipate that this protocol will attract considerable interests from the material scientists and biologists, who rely on AD drug screening and pharmacological study day-in and day-out in their laboratories.
- Perrin, R. J., Fagan, A. M. & Holtzman, D. M. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature 461, 916-922 (2009).
- Ross, C. A. & Poirier, M. A. Protein aggregation and neurodegenerative disease. Nat. Med. 10, 10-17 (2004).
- Gremer, L. et al. Fibril structure of amyloid-β(1-42) by cryo-electron microscopy. Science 358, 116-119 (2017).
- Fu, W. et al. Rational design of near-infrared aggregation-induced-emission-active probes: in situ mapping of amyloid-β plaques with ultrasensitivity and high-fidelity. J. Am. Chem. Soc. 141, 3171-3177 (2019).
- Yan, C. et al. Preparation of near-infrared AIEgen-active fluorescent probes for mapping amyloid-β plaques in brain tissues and living mice. Protoc. doi: 10.1038/s41596-022-00789-1 (2023).
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in