Programming Bacterial Communication for Teamwork in a Test Tube
Published in Microbiology, Cell & Molecular Biology, and Plant Science
What is Biocomputing?
Synthetic biology offers new solutions to agriculture, healthcare, manufacturing, and environmental challenges. As a branch of synthetic biology, biocomputing is the use of living cells, like bacteria, to process information—similar to how electronic computers work but using biological components instead of circuits. We engineer genetic “programs” that allow cells to detect signals, make decisions, and respond in specific ways. This technology has exciting applications, such as bacteria that sense environmental pollution 1, plants that activate defenses against stress 2 and microbes that deliver medicine to precise locations in the body3.
Challenges of Biocomputing and How to Overcome Them
Biocomputing faces several hurdles, such as the burden it puts on cells, unintended interference between genetic parts, and difficulty in coordinating complex tasks. Traditionally, synthetic biologists have used proteins to control gene activity, but this approach has limitations. CRISPR interference (CRISPRi) provides an interesting alternative by using guide RNA to precisely block gene expression, reducing unwanted interactions and energy demands on the cells variants.
Another way to improve biocomputing is by distributing tasks among different groups of cells, similar to how different components in a computer work together. However, making these cells communicate effectively is a challenge. Scientists have tried using chemical signals, but these can only send one type of message at a time. A more versatile approach involves using bacteria’s natural ability to transfer DNA, allowing cells to send and receive multiple instructions more efficiently—like replacing simple radio signals with high-speed internet for biological communication.
Using ‘DNA Messaging’ for Multicellular Computing
We have developed a new way for bacteria to communicate and work together, inspired by how electronic circuits process information. Building on the work of Ortiz and Endy4, our approach combines CRISPRi along with M13 phages (a type of virus that infect bacteria!) to transfer genetic instructions, in the form of single-guide RNA (sgRNA), between bacterial cells. These ‘instructions’ help control gene activity in the receiver cells.
By carefully designing this system, we can control when and which genetic messages are sent between different bacterial groups. This allows us to build biological logic circuits—similar to computer logic gates—that can efficiently process information. Compared to traditional chemical-based communication methods, our system is can produce a response within 4 hours. This work provides a powerful new method for programming bacterial communities to perform complex tasks. By controlling the expression of gene VIII that constitutes the major phage capsid protein (the parts that form the shell of the phage), we were able to control when and how genetic messages are passed between bacteria (Figure 1). This let us assign specific computing tasks—like building 2-input and more complex 4-input logic gates—to different groups of bacteria.
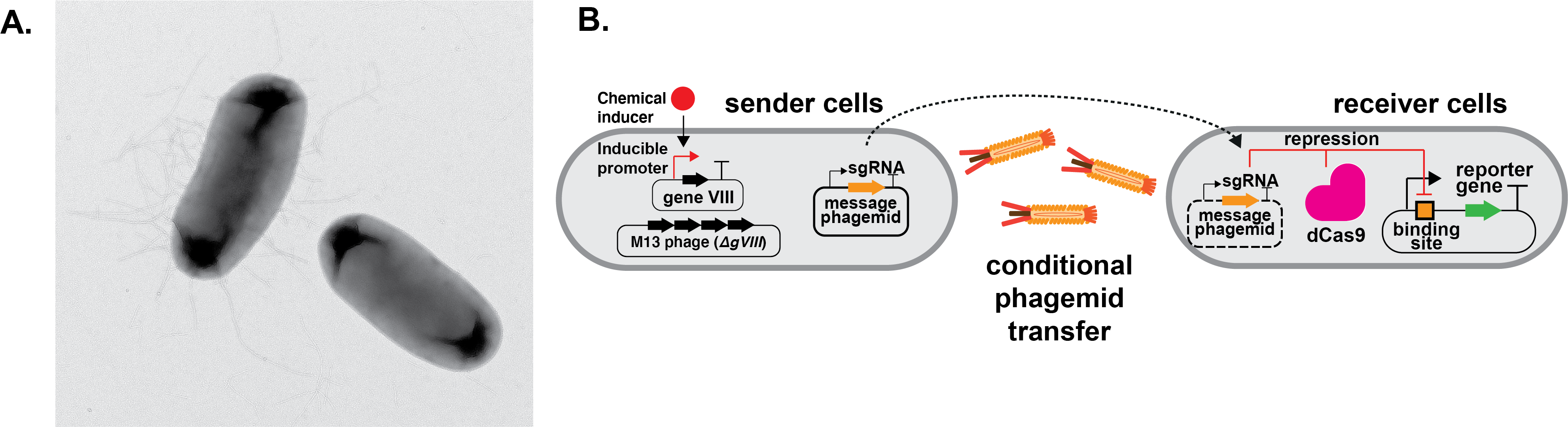
Figure 1. Intercellular communication using M13 phage and CRISPR-based gene regulation. A. Transmission electron microscopy image of a phagemid sender cell and a receiver cell during phage transfer. B. Intercellular communication is achieved through the exchange of genetic material between sender and receiver cells mediated by M13 phage transfer. M13 phage particles contain a plasmid with a M13 packaging signal (phagemid) that encodes the DNA message, in this case a single guide RNA. Phage particles are only produced when a chemical inducer is added. This regulation is achieved by placing gene VIII under the regulation of an inducible promoter.
What were the challenges in this project?
One big challenge was figuring out which part of the phage we could control to make its message-sending ability switch on or off. We tried almost every capsid protein, but most did not really affect message production, or they were needed in such tiny amounts that they were nearly impossible to regulate. After a lot of trial and error, we finally found our star player: protein VIII, expressed from gene VIII. That one did the trick!
Another fun twist was that there was another research group working on a similar idea 5. So yes, it felt a bit like a scientific race! Luckily, our approaches were different, and in the end, our results turned out to complement each other nicely. Their work helped validate ours, and we hope ours does the same for them.
Of course, like any good scientific journey, the reviewers gave us a long list of things to fix. While it felt overwhelming at times, their feedback definitely helped us improve the paper and make the story stronger overall.
So, what is next?
In this study, all the genetic messages were sent to the same type of receiver cell. This setup helped us fine-tune our system, which uses phagemid-based message delivery combined with CRISPR tools to control gene activity. We showed that this method is more sensitive than traditional chemical-based communication and used it to build complex logic gates—like four-input AND and NAND gates—within a community of E. coli.
Looking ahead, we plan to push this further by not just distributing the processing of the input signals across different cells, but also spreading out the output signals. This will open the door to even more advanced “biocomputing” tasks, such as performing basic math operations like addition and multiplication using living cells. With current tools for designing CRISPR guide RNAs and the ability to use many of them at once 6, we believe this system can be scaled up for much more complex tasks.
We are also excited about combining our system with other types of cell communication, like chemical signals or other forms of DNA sharing, to create even more versatile tools. In summary, our work shows that using phagemids and CRISPR together is a powerful way to program groups of bacteria to perform complex tasks. This could one day lead to new technologies in areas like biosensing, pollution cleanup, smart drug delivery, and more.
References:
- Wang, B., Barahona, M. & Buck, M. A modular cell-based biosensor using engineered genetic logic circuits to detect and integrate multiple environmental signals. Biosens Bioelectron 40, 368–376 (2013).
- Anderson, C. E., Ferreira, S. S. & Antunes, M. S. Integration of multiple stress signals in plants using synthetic Boolean logic gates. Plant Physiol 192, 3189–3202 (2023).
- Chien, T. et al. Enhancing the tropism of bacteria via genetically programmed biosensors. Nat Biomed Eng 6, 94–104 (2022).
- Ortiz, M. E. & Endy, D. Engineered cell-cell communication via DNA messaging. J Biol Eng 6, 16 (2012).
- Pujar, A. et al. Phage-mediated intercellular CRISPRi for biocomputation in bacterial consortia. Nucleic Acids Res 53, gkae1256 (2025).
- Shaw, W. M. et al. Inducible expression of large gRNA arrays for multiplexed CRISPRai applications. Nat Commun 13, 4984 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in