Prolonged indoleamine 2,3-dioxygenase-2 (IDO2) activity and associated cellular stress in post-acute sequelae of SARS-CoV-2 infection(PASC, or long COVID)
Published in Social Sciences and General & Internal Medicine
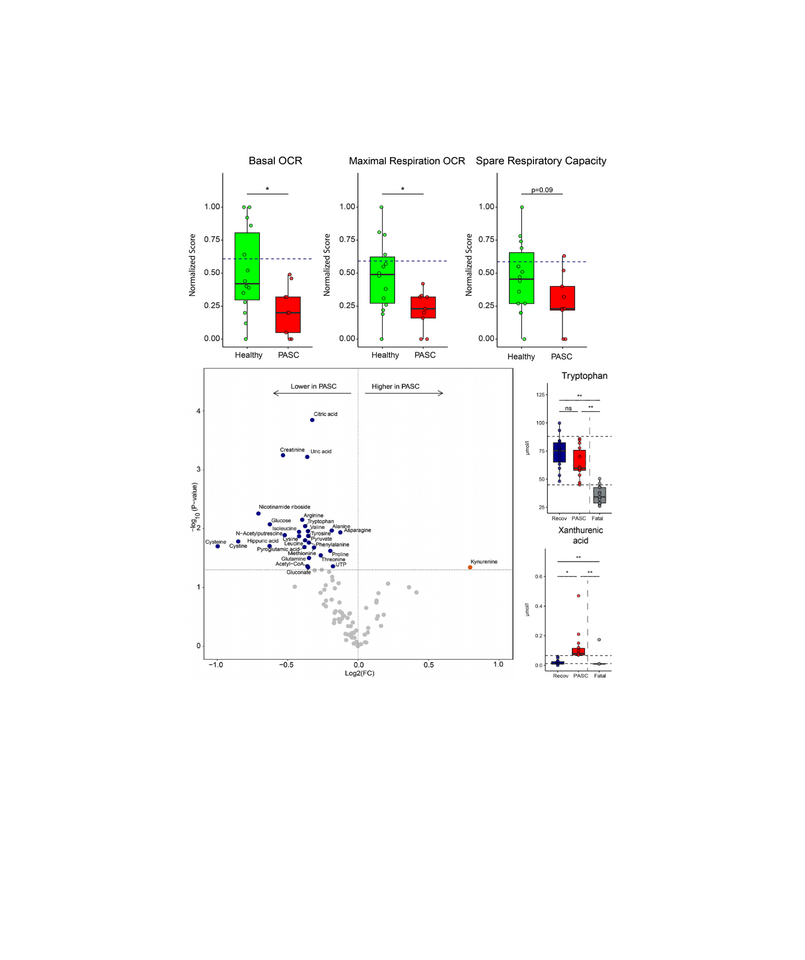
Post-acute sequela of SARS-CoV-2 infection (PASC, also long COVID) encompass fatigue, post-exertional malaise and cognitive problems. The abundant expression of the tryptophan-catabolizing enzyme IDO2 in fatal/severe COVID-19, led us to determine, in an exploratory observational study, whether IDO2 is expressed and active in PASC, and may correlate with pathophysiology. Plasma or serum, and peripheral blood mononuclear cells (PBMC) were obtained from well-characterized PASC patients and SARS-CoV-2-infected individuals without PASC. We assessed tryptophan and its degradation products by UPLC-MS/MS. IDO2 activity, its potential consequences, and the involvement of the aryl hydrocarbon receptor (AHR) in IDO2 expression were determined in PBMC from another PASC cohort by IHC for IDO2, IDO1, AHR, kynurenine metabolites, autophagy, and apoptosis. These PBMC were also analyzed by metabolomics and for mitochondrial functioning by respirometry. IHC was also performed on autopsy brain material from two PASC patients. IDO2 is expressed and active in PBMC from PASC patients, as well as in brain tissue, long after SARS-CoV-2 infection. This is paralleled by autophagy, and in blood cells by reduced mitochondrial functioning, reduced intracellular levels of amino acids and Krebs cycle-related compounds. IDO2 expression and activity is triggered by SARS-CoV-2-infection, but the severity of SARS-CoV-2-induced pathology appears related to the generated specific kynurenine metabolites. Ex vivo, IDO2 expression and autophagy can be halted by an AHR antagonist.
Evidence before this study:
Earlier studies1, 3 indicated that the degradation of tryptophan to kynurenine and further breakdown products was enhanced in SARS-CoV-2 infected individuals, likely caused by the interferon-induced tryptophan-catabolizing enzyme IDO1, which typically is induced by viral infections. Rather than IDO1, our immunohistochemical analyses of lung, heart, brain and blood cells from patients with fatal/severe COVID-19 showed extensive expression and activity of the otherwise rarely expressed IDO2. This was associated with reduced systemic tryptophan and enhanced breakdown products (among others, kynurenine and the neuro-/cytotoxic quinolinic acid and 3OH-kynurenine) and, spatially, with autophagy and apoptosis. This led us to study IDO2 expression in patients with PASC.
Added value of this study2:
IDO2 is expressed in blood cells from PASC patients and manifests itself long after the initial infection by SARS-CoV-2. IDO2 expression is driven by the AHR and coincides with an altered cellular metabolism,reflected by attenuated mitochondrial functioning and autophagy. SARS-CoV-2 infection induces IDO2 expression, but the resulting pathology appears related to the specific kynurenine breakdown products that are generated. In contrast to fatal/severe COVID-19 we found marked autophagy and limited apoptosis in PASC. This was confirmed in autopsy brain tissue from PASC patients. All PASC patients experienced an increase in plasma xanthurenic acid levels, while only some patients exhibited significantly elevated levels of 3-hydroxy anthranilic acid.
Implications of all the available evidence:
Together these findings imply the AHR-IDO2-kynurenine pathway in SARS-CoV-2-induced pathology and that of PASC in particular. Further studies into IDO2 activity and the downstream kynurenine products at the cellular level in a larger PASC cohort, and its association with quantitative parameters of symptoms would substantiate these finding. An explorative trial in PASC to interfere with the AHR-IDO2 pathway is warranted. As there are no potent inhibitors of IDO2 available for use in humans, an AHR antagonist is a likely therapeutic option.
References:
1. Guo, Lihui, Bernadette Schurink, Eva Roos, Esther J. Nossent, Jan Willem Duitman, Alexander PJ Vlaar, Paul van der Valk et al. "Indoleamine 2, 3‐dioxygenase (IDO)‐1 and IDO‐2 activity and severe course of COVID‐19." The Journal of pathology 256, no. 3 (2022): 256-261.
https://pathsocjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/path.5842
https://pathsocjournals.onlinelibrary.wiley.com/doi/full/10.1002/path.5842
2. Guo, Lihui, Brent Appelman, Kirsten Mooij-Kalverda, Riekelt H. Houtkooper, Michel van Weeghel, Frédéric M. Vaz, Annemiek Dijkhuis et al. "Prolonged indoleamine 2, 3-dioxygenase-2 activity and associated cellular stress in post-acute sequelae of SARS-CoV-2 infection." EBioMedicine 94 (2023).
https://www.thelancet.com/action/showPdf?pii=S2352-3964%2823%2900294-3
https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(23)00294-3/fulltext
3.Schurink, Bernadette, Eva Roos, Teodora Radonic, Ellis Barbe, Catherine SC Bouman, Hans H. de Boer, Godelieve J. de Bree et al. "Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study." The Lancet Microbe 1, no. 7 (2020): e290-e299. https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(20)30144-0/fulltext




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in