Pushing the limit of water flux and ion sieving in nanofiltration membranes
Published in Materials

Efficient water-ion separation is essential for seawater desalination and industrial water purification. The ideal situation involves selectively sieving ions while allowing water molecules to move rapidly. However, conventional molecular transport theory presents a challenge as ultrafast water flux and effective ion sieving appear to be conflicting factors. Achieving effective ion sieving typically requires reducing pore size, which can impede water flux in hydrophilic membranes. In 2016, Professor A. K. Geim's team at the University of Manchester discovered unexpectedly fast water transport, reaching speeds of up to 1 meter per second, through atomic-scale graphene capillaries. This phenomenon was attributed to high capillary pressures and large slip lengths (Nature 2016, 538, 222-225). However, the graphene capillary devices used in this study were fabricated using photolithography, making them challenging to apply in water purification processes. This discovery has prompted us to consider whether we can utilize atomic-scale graphene capillaries for ion sieving through size exclusion, all while maintaining rapid water transport in a scalable nanofiltration (NF) membrane.
The challenge lies in fabricating NF membranes with atomic-scale graphene capillaries. Reduced graphene oxide (rGO)-based membranes are promising candidates, but they often face the issue ofrGO restacking, which seals the atomic-scale graphene capillaries. Therefore, we propose a redesign of the rGO structure by creating new heterostructures, known as graphitic nano-islands on rGO nanosheets, and utilizing the nano-islands as pillars to construct atomic-scale graphene capillaries in NF membranes. The key to success is controlling the atomic height of the nano-islands on rGO nanosheets. This goal has been successfully achieved by a catalytic reaction involving Ni ions, p-phenylenediamine (pPD), and oxygenated functional groups on graphene oxide (GO) nanosheets, resulting in the formation of Ni-pPD monolayer-islands distributed uniformly on rGO nanosheets (Fig. 1a). Upon restacking of these heterostructures, atomic-scale graphene capillaries were generated as confirmed by pore size distribution (PSD) analysis (Fig. 1b), which clearly demonstrates three sharp and distinctive peaks below 1 nm, with the strongest peak sitting at ~0.6 nm. The membranes made of the restacked Ni-pPD@rGO nanosheets have then been fabricated by vacuum filtration, followed by the controlled evaporation under external pressures. These membranes were denoted as metal-organic (Ni-pPD) pillared, graphene-based membranes (MOGMs). As consistent with previous reports, GO membranes (GOMs) contain hydrophilic capillaries and the contact angle obtained for GOMs is 44.5° (Fig. 1c). In contrast, the contact angle for MOGMs is 84°, indicating that MOGMs are full of atomic-scale graphene capillaries. The optimized structure computed by density-functional-theory (DFT) calculations (Fig. 1d) demonstrates that two monolayer islands of Ni-pPD are pillared between two rGO through π-π stacking, generating the free space of 2.6 Å and 6.2 Å. Therefore, unlike activated graphene/carbons that normally have a continuous size distribution of micropores, the pore structure in MOGMs resembles the structural characteristics of molecular sieves with the pathways to conduct water molecules illustrated in Fig. 1e, manifesting the delicacy of this synthesis strategy.
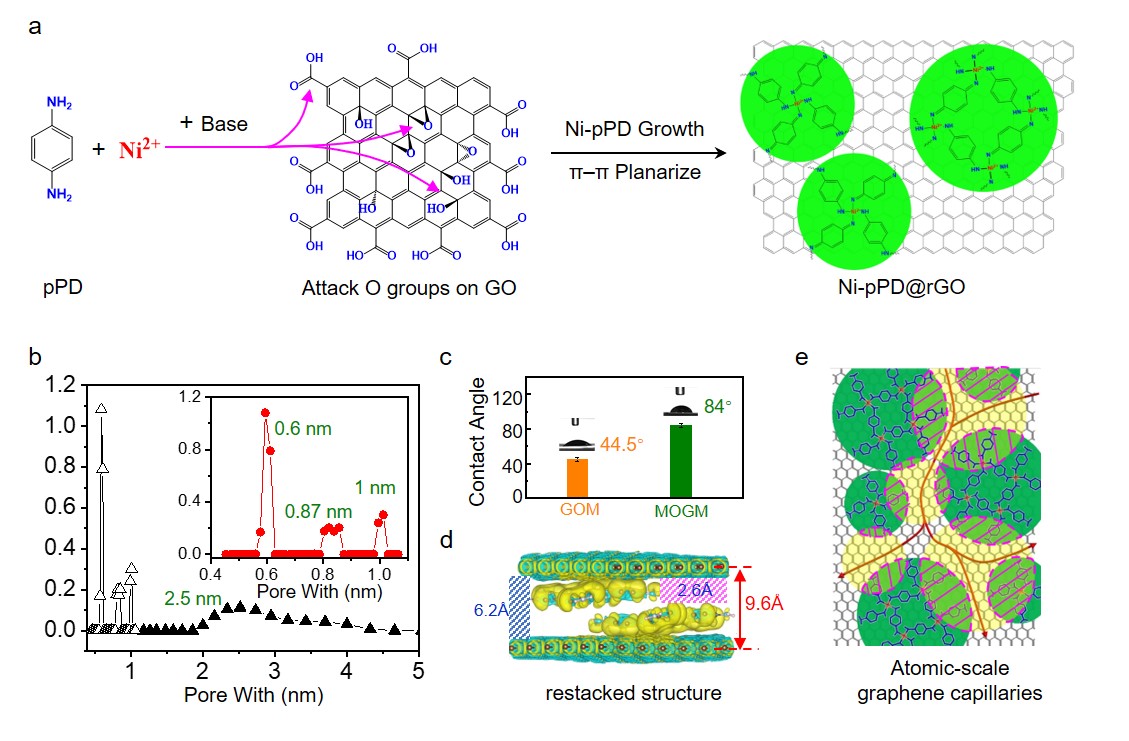
Fig. 1| Synthesis and characterization of Ni-pPD@rGO nanosheets and MOGMs. a Synthesis route of Ni-pPD@rGO nanosheets. b Pore size distribution of MOGMs. c Water contact angle and XRD spectra of MOGMs and GOMs. d The optimized structure of restacked Ni-pPD@rGO nanosheets computed by DFT, with the interlayer spacing labelled by a red arrow and the free space marked by slash lines. e Schematic of the sub-nano pathways to conduct water molecules in MOGMs. Green: bottom Ni-pPD nano-islands; Yellow: top Ni-pPD nano-islands; Pink-shaded frames: fully blocked regions by the restacking of two Ni-pPD nano-islands.
What frustrated us at the beginning was that, when we put MOGMs in a U-shaped permeation cell (Fig. 2a), no water flux could be detected for more than 1 day of operation, under the osmotic pressure of 7.4 bar (0.1 M MgCl2 solution) and with no height difference between two compartments. At first, we suspected that the atomic-scale graphene capillaries may be too small for any molecules to transport. Unexpectedly, when exerting a small hydrostatic pressure as low as 7.8×10–3 bar on the feed side (indicated as a dashed line in Fig. 2b), the water flow suddenly shoots up to 0.71 L m–2 h–1. The water flux keeps increasing up to 2.5 L m–2 h–1 at 9.8×10-3 bar (“ON” state in Fig. 2b), which is about 10 times faster than that obtained by GOMs under the same experimental conditions. At the same time, the ion permeation rates of Mg2+ keep at very low values (Fig. 2b), around 3 orders of magnitude lower than those of GOMs. More importantly, water flow could be turned on and off reversibly (Fig. 2b), by adjusting the hydrostatic pressure higher and lower than the gating pressures (PG: ~10–2 bar), respectively, revealing the successful establishment of a new gating mechanism for water-ion separation.
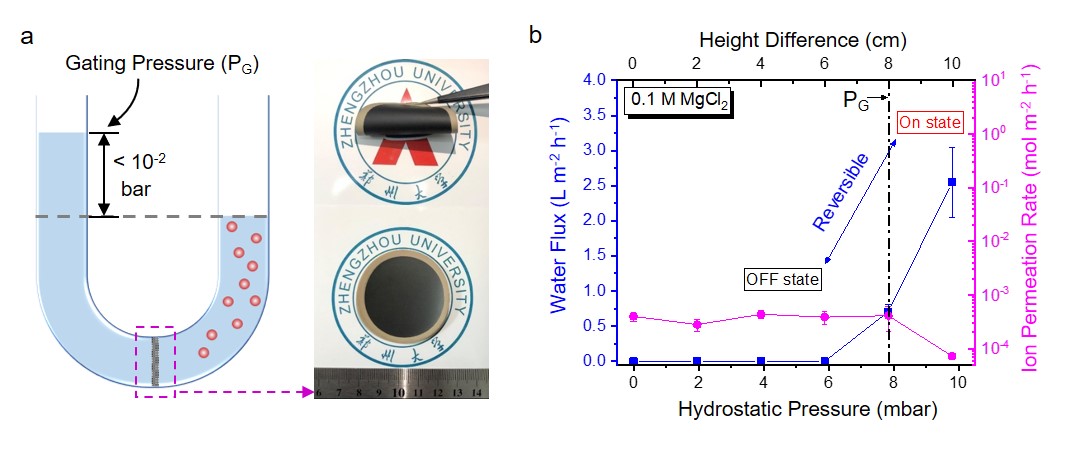
Fig. 2| Anomalous permeation through MOGMs. a Schematic of the experimental setup with inserted optical graphs of MOGMs. b Change of water flux and ion permeation rates with hydrostatic height differences in the centimeter range in the case of 0.1 M MgCl2 as the draw solution. The gating pressure is 7.8×10–3 bar (labelled as a dashed line). The reversible water on/off switch, ultrafast water flux, and effective ion sieving for Mg2+ ions are also illustrated. All the error bars in this figure represent the standard deviations for at least three measurements.
In order to theoretically reveal the new gating mechanism, we performed in-situ Fourier-transform infrared spectroscopy (in-situ FTIR) and found that H-bonded water network formed within the atomic-scale graphene capillaries is stronger than that in the liquid water. First principles/molecular dynamics (MD) simulation calculation shows that the strong H-bonded water network is in the form of 2D crystal-like water nanosheets. With this structure in the highly-confined space, the whole water-intercalated graphene capillary becomes a solid, suppressing water and ion diffusion in “OFF” state (Fig. 3a). When applying a hydrostatic pressure in the “ON” state, a shear force will be applied onto the ordered water nanosheet, and the water transport mechanism is by analogy with high-speed, diffusion-free Newton’s cradle-like Grotthus conduction (Fig. 3b). In this way, a new water molecular gating mechanism has been established for the anomalous permeation behavior being observed in this work.
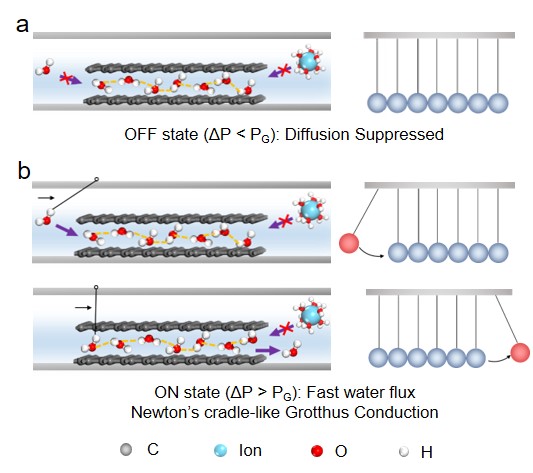 Fig. 3| Microscopic illustrations of water molecular gating mechanism. a “OFF” state. b “ON” state with Newton’s cradle-like Grotthus Conduction originated from highly-connected hydrogen-bonded network.
Fig. 3| Microscopic illustrations of water molecular gating mechanism. a “OFF” state. b “ON” state with Newton’s cradle-like Grotthus Conduction originated from highly-connected hydrogen-bonded network.
This work enriches our fundamental understandings in the correlation between the pore structure and the permeation performance at the atomic scale. In a broader context, the discovered water molecular gating mechanism originated from liquid-solid-liquid, phase-changing molecular transport not only offers insights into preventing inevitable diffusion in the liquid state, but also provides solutions for improving slow transport kinetics in the solid state. These findings have broad applications across various fields related to molecular transport, including water desalination, purification, life sciences, nanofluidics, ion transistors, energy conversion, energy storage, and beyond.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in