β-Receptor blocker enhances the anabolic effect of PTH after osteoporotic fracture
Published in General & Internal Medicine and Surgery
The growing challenge of osteoporotic fractures
As the global population ages, the prevalence of osteoporotic fractures is on the rising, posing a dramatic clinical challenge. Patients with osteoporotic fractures not only struggle with fracture healing, but also face an increased risk of subsequent fractures.1 Previous fractures are considered one of the most reliable predictors of the risk of next fractures, known as “imminent fracture risk”,2highlighting the importance of timely treatment for systemic bone loss after fracture.
An osteoporotic fracture is like one disaster after another. While treating the current fracture is crucial, preventing subsequent fractures holds greater significance. Current therapeutic strategies to prevent systemic bone loss and subsequent fractures are limited. The use of anti-osteoporosis drugs after fracture is controversial due to their impact on bone remodelling, which is essential for fracture healing. The battle against osteoporotic fractures, a looming concern in aging population, demands new solutions.
Combating osteoporotic fractures: a new dawn with PTH and β-receptor blocker
Bone, traditionally viewed as the rigid framework supporting the body and a reservoir of calcium and phosphorus, has recently been shown to have the properties of an endocrine organ, intricately regulated by autonomic nerves and hormones.3 Bone metabolism is controlled by a complex interplay of various signaling pathways. It is well established that sympathetic nerves suppress bone formation via osteoblast β-adrenergic receptor (βAR) signaling.3 Interestingly, there is a functional interaction between β2AR signaling and parathyroid hormone 1 receptor (PTH1R), with β2AR deficiency hindering both PTH-induced increases in bone formation and resorption.4 These findings inspire combined drug intervention in bone metabolism disorders. Given the sympathetic stress after fracture, we propose that combination of PTH and propranolol (a nonselective βAR antagonist) may contribute to a better therapeutic effect on systemic bone loss after osteoporotic fracture.
To examine the effects of PTH and β-receptor blocker (propranolol) on systemic bone loss after osteoporotic fracture, we generated fracture models in mice with ovariectomy (OVX)-induced postmenopausal osteoporosis. μCT analysis of femurs revealed that fracture resulted in significantly decreased trabecular bone mass in OVX mice. Propranololadministration mildly but significantly increased the trabecular bone volume fraction and trabecular thickness (Fig. 1a, c, d), and PTH dramatically increased cortical bone and trabecular bone volumes (Fig. 1a-f). Importantly, the combined treatment with PTH and propranolol increased cortical bone and trabecular bone mass to a greater extent than treatment with either agent alone (Fig. 1a-f). Compared to the vehicle group, the trabecular bone volume fraction was 54% greater in mice treated with PTH alone, and was 93% greater in mice treated with the combination of PTH and propranolol, indicating that propranolol increased the osteogenic effect of PTH by approximately 80% in mice with osteoporotic fractures.
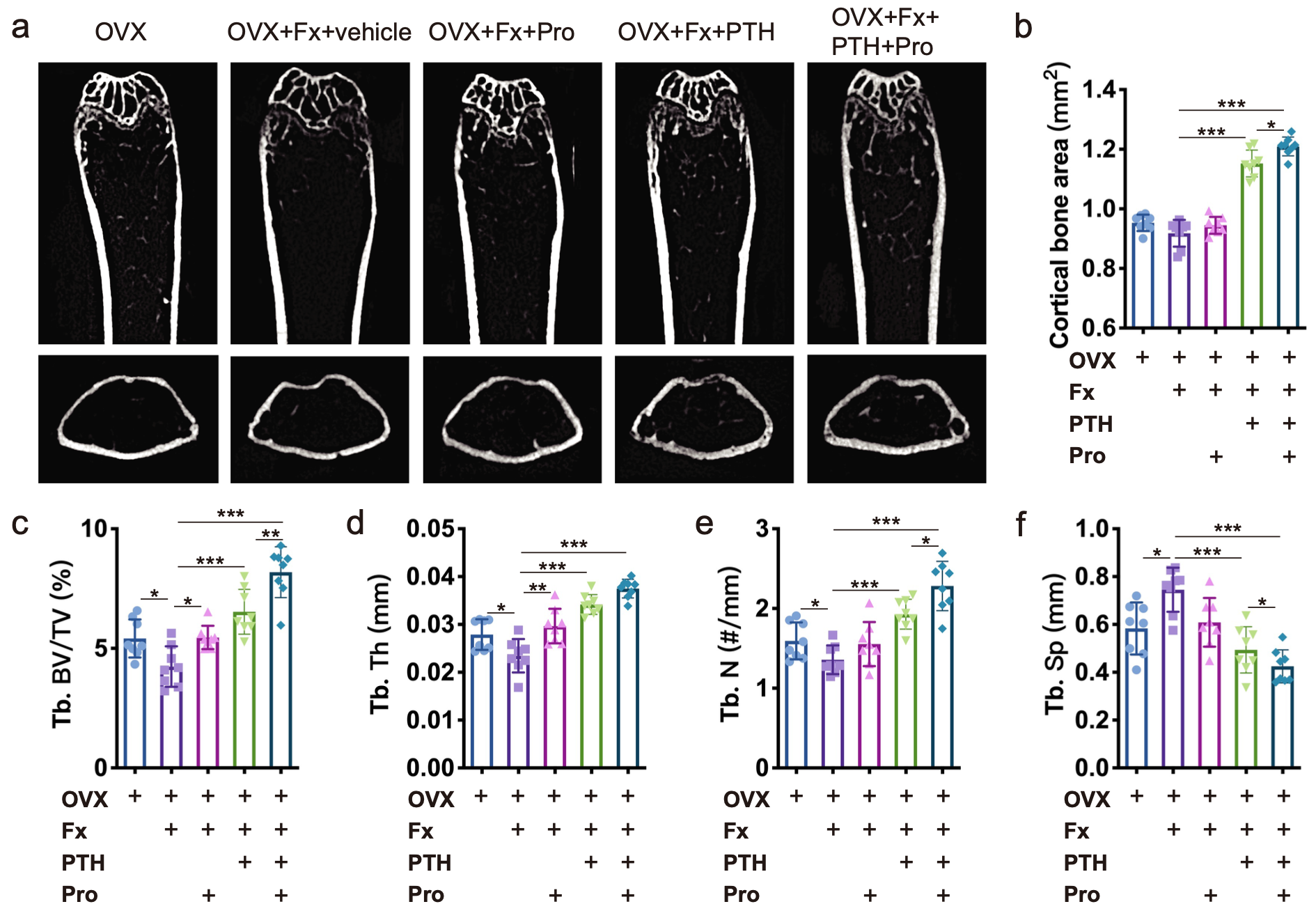
Figure 1. a Representative μCT images of femurs from OVX, OVX + Fx (fracture), and OVX + Fx mice treated with Pro (propranolol), PTH or PTH + Pro. b-f Quantitative μCT analysis of the cortical bone area (b), trabecular bone volume fraction (Tb. BV/TV; c), trabecular bone thickness (Tb. Th; d), trabecular bone number (Tb. N; e), and trabecular bone separation (Tb. Sp; f)
Furthermore, mice administered PTH showed significantly increased BV and BV/TV in calluses compared to the vehicle group, whereas higher BV and BV/TV in calluses were observed in mice treated with PTH + propranolol than in those treated with propranolol or PTH alone (Fig. 2a-c), indicating that propranolol enhances the efficacy of PTH in promoting fracture healing.
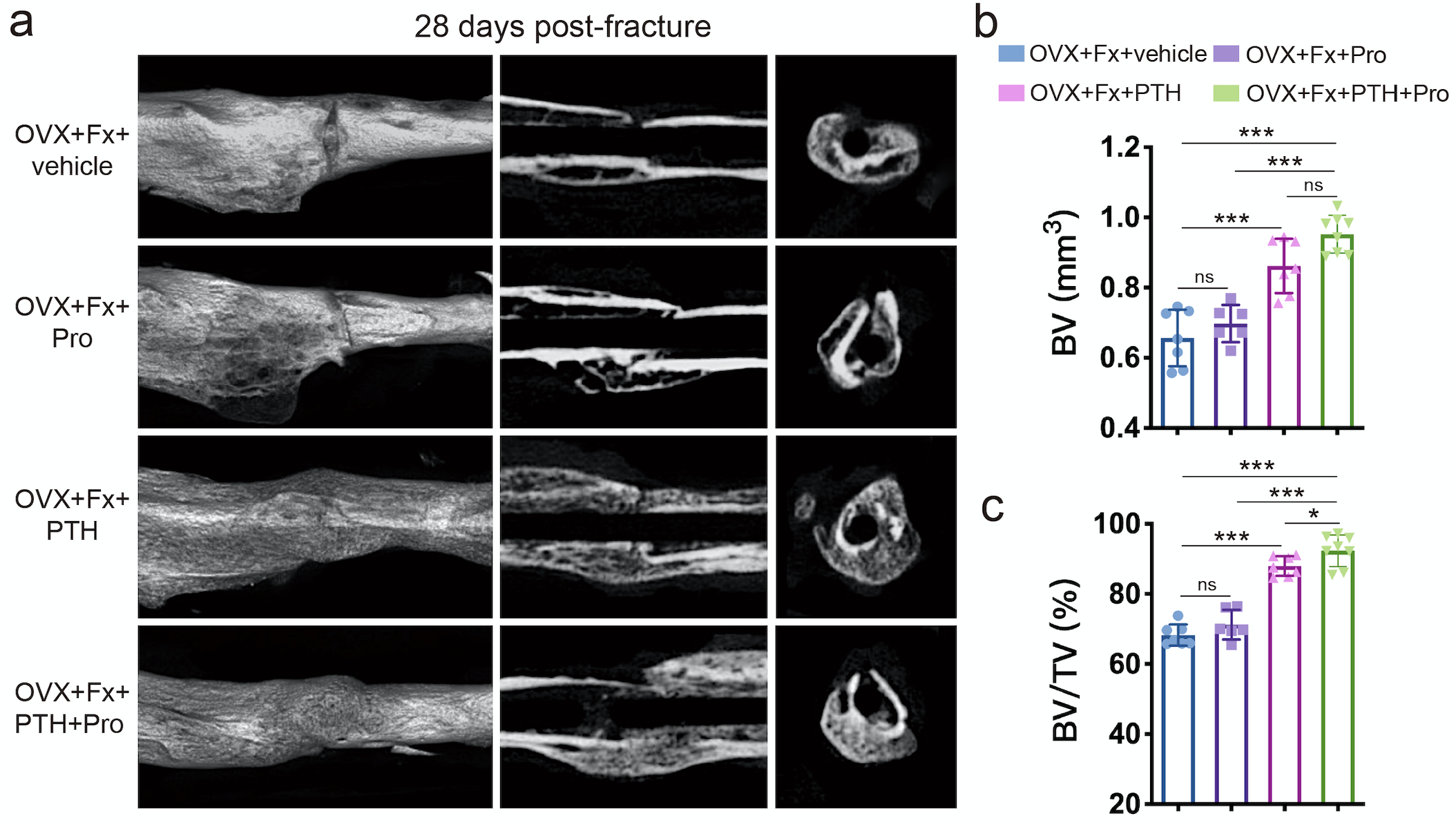
Figure 2. a μCT 3D reconstructions and axial cross-sectional and coronal cross-sectional images of fractured tibias from OVX mice treated with PTH, Pro, PTH + Pro or vehicle on day 28 after fracture. b, c Quantitative analysis of the bone volume (BV) and the bone volume fraction (BV/TV) of the calluses. n=6-8 per group.
Both plasma PTH and NE levels exhibit diurnal rhythms, and circadian clock genes play an essential role in regulating bone metabolism.5 We further examined the expression of all core clock genes and found that NE significantly inhibited iPTH-induced the expression of Bmal1 (a core component of the circadian clock loop). We further demonstrated that NE-activated βAR signaling suppresses PTH-induced osteogenesis and enhances the effect of PTH-induced osteoblasts on osteoclast formation via a Clock-dependent mechanism, which is reversed by propranolol. Specifically, NE-βAR signaling inhibits PTH-induced RUNX2 expression and osteoblast differentiation by regulating the circadian clock gene BMAL1 (Figure 3).
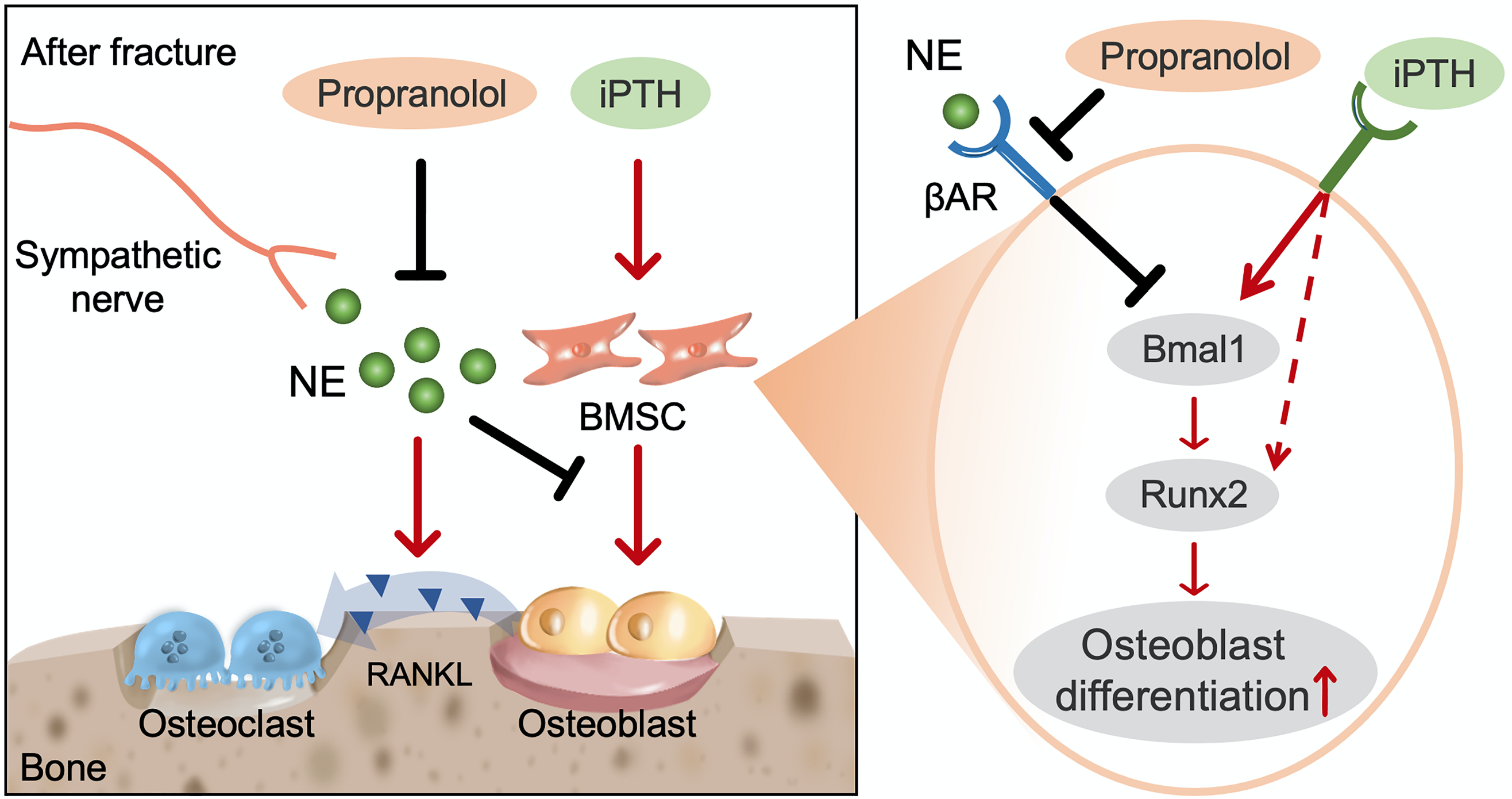
Figure 3. Schematic diagram showing the cellular and molecular mechanism by which propranolol augments the anabolic effect of PTH on systemic bone loss after fracture
Conclusion and Outlook
In summary, our study shows that β-receptor blocker dramatically enhances the anabolic effect of PTH in preventing systemic bone loss following osteoporotic fracture by blocking the negative effects of sympathetic signaling on PTH anabolism, which may provide a novel therapeutic strategy for the treatment of systemic bone loss after osteoporotic fracture. It's a potential game changer in the treatment of osteoporotic fractures, signaling a more personalized and effective therapeutic option. Moving forward, We are currently conducting relevant clinical trials to validate these findings and explore the optimal timing and dosing for therapy.
References
1. Compston, J. E., McClung, M. R. & Leslie, W. D. Osteoporosis. Lancet 393, 364-376, doi:10.1016/s0140-6736(18)32112-3 (2019).
2. Wong, R. M. Y. et al. The imminent risk of a fracture-existing worldwide data: a systematic review and meta-analysis. Osteoporos Int 33, 2453-2466, doi:10.1007/s00198-022-06473-0 (2022).
3. Elefteriou, F. Impact of the Autonomic Nervous System on the Skeleton. Physiol Rev 98, 1083-1112, doi:10.1152/physrev.00014.2017 (2018).
4. Hanyu, R. et al. Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proc Natl Acad Sci U S A 109, 7433-7438, doi:10.1073/pnas.1109036109 (2012).
5. Luo, B. et al. Circadian rhythms affect bone reconstruction by regulating bone energy metabolism. J Transl Med 19, 410, doi:10.1186/s12967-021-03068-x (2021).
Follow the Topic
-
Bone Research

This journal highlights the breakthrough discoveries in basic and clinical aspects of bone biology, pathophysiology and regeneration, as well as other significant findings related to bone.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in