Relationship among DDR gene mutations, TMB and PD-L1 in solid tumor genomes identified using clinically actionable biomarker assays
Published in Cancer, Cell & Molecular Biology, and General & Internal Medicine
Background
Genomic instability has been recognized as a critical factor in the development and progression of cancer. DNA damage response (DDR) pathways and repair mechanisms play a pivotal role in maintaining genomic stability. Dysregulation or aberrations in DDR components can lead to defective DNA repair, contributing to genomic instability and the onset of cancer, such as ovarian cancer (OC), bladder cancer (BC), colorectal cancer (CRC), prostate cancer (PC), and non-small cell lung cancer (NSCLC).
Recently, DDR genes have attracted considerable attention as predictive and prognostic markers, indicators of therapeutic response, and targets for cancer treatment. Genomic alterations (GAs) in genes involved in homologous recombination (HR) DNA repair, such as BRCA1/2, are particularly relevant. These alterations indicate HR deficiency and can identify patients likely to respond to treatments like poly (ADP-ribose) polymerase (PARP) inhibitors, with implications for breast, ovarian, prostate, and other cancers. Beyond BRCA1/2, numerous HR GAs have been identified in various cancers, presenting promising druggable targets.
Emerging evidence suggests that combining DDR inhibitors with immunotherapy produced synergistic effects in tumors harboring DDR GAs. This highlights the importance of exploring DDR GAs and leveraging established predictive biomarkers like tumor mutational burden (TMB), microsatellite instability (MSI), and PD-L1 immunohistochemistry (IHC) expression.
Objectives
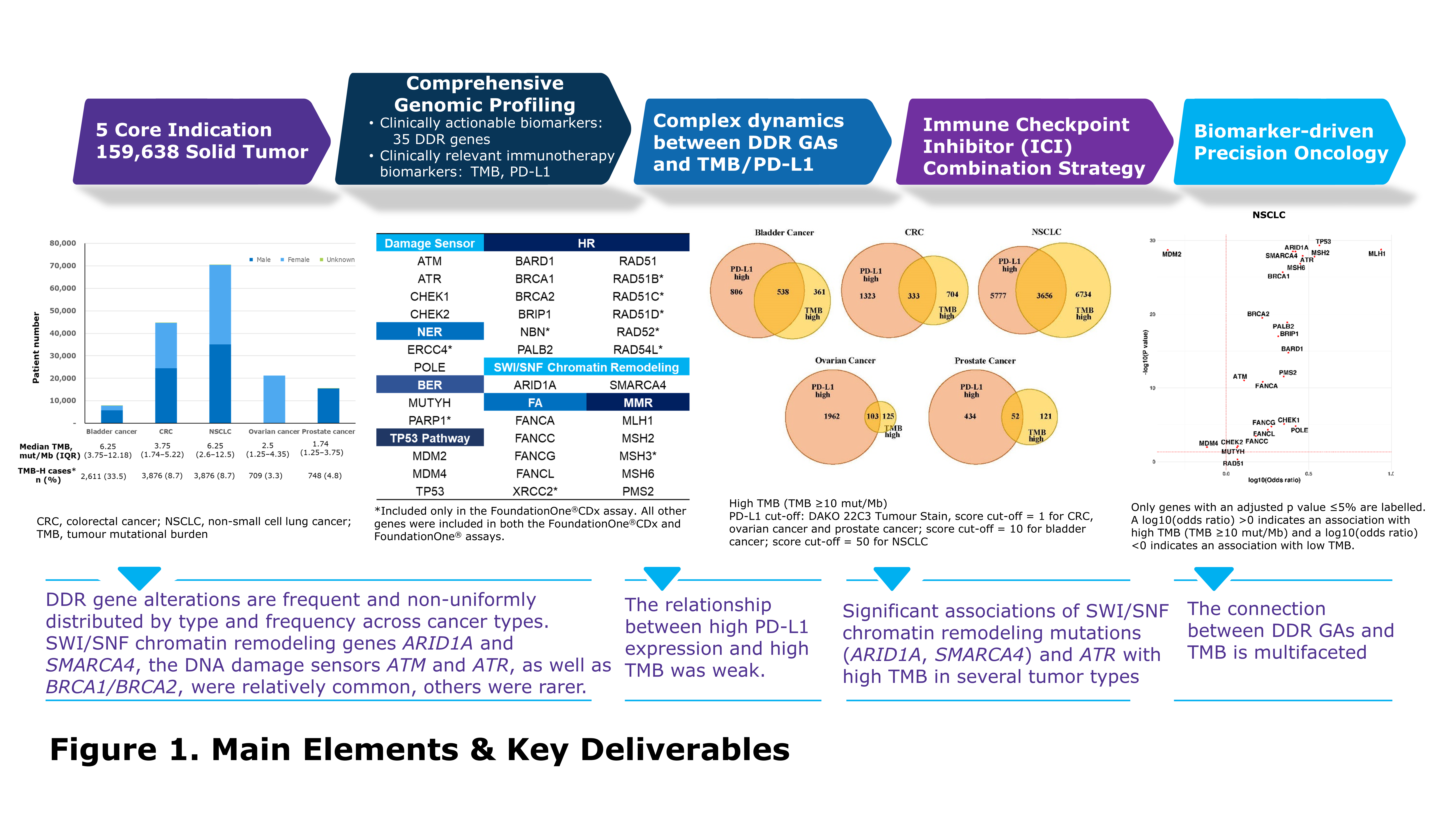
Real-world genomic data mining via the Foundation Medicine FoundationInsights® web platform, focusing on 35 DDR genes, was conducted to investigate the relationship between DDR GAs and cancers. These selected genes encompass various DNA damage and response functions or pathways, including base excision repair, damage sensing, HR, mismatch repair, nucleotide-excision repair, SWI/SNF chromatin remodeling, Fanconi anemia, and the TP53 pathway. The analysis centered on five tumor types—BC, CRC, NSCLC, OC, and PC to explore associations with clinically relevant immunotherapy biomarkers (TMB, PD-L1) and to pinpoint opportunities for combination therapy.
Key Findings (Figure 1)
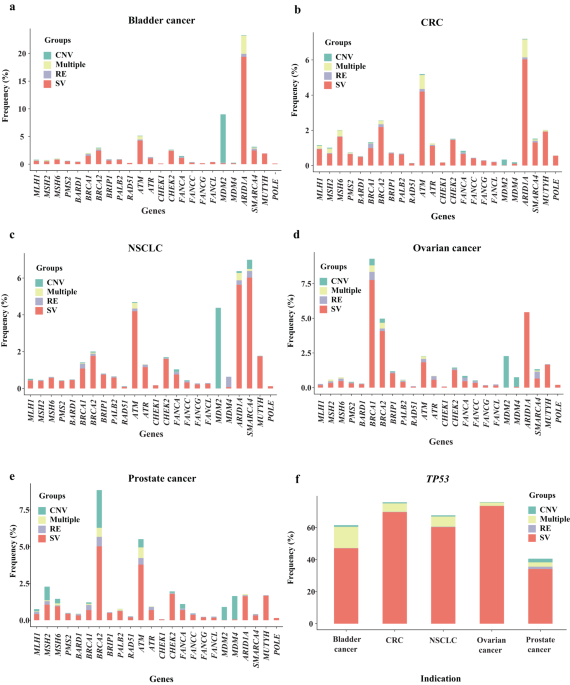
- The genomic landscape of 35 DDR genes, including somatic and germline mutations, across 159,638 solid tumors was analyzed. It became apparent that a significant proportion of these tumors harbored DDR gene alterations, with TP53 being the most frequently mutated across all five tumor types. This analysis builds upon previous research, providing a more comprehensive examination of DDR gene alterations in selected cancer types.
- While some DDR gene alterations, such as those in the SWI/SNF chromatin remodeling genes ARID1A and SMARCA4, the DNA damage sensors ATM and ATR, as well as BRCA1/BRCA2, were relatively common, others were rarer. Notably, mutations in base excision repair, mismatch repair, Fanconi anemia, and nucleotide excision repair genes were infrequent. However, HR genes, especially in ovarian and prostate cancers, exhibited higher mutation rates, illuminating the cancer-specific genomic landscape of these tumor types.
- To elucidate the clinical relevance, associations of DDR gene alterations with TMB and PD-L1 status were investigated.
-
- A complex relationship emerged between DDR GAs and TMB (Figure 1). Focus was placed on genes with a statistically significant strong association with TMB, revealing associations between mutations in multiple mismatch repair genes and TMB in all tumor types (CRC: MLH1, MSH2, MSH6, and PMS2; NSCLC: MLH1 and MSH2; OC: MSH2 and MSH6; PC: MLH1, MSH2, and MSH6), except BC. There were also significant associations between mutations in the nucleotide excision repair gene POLE and TMB in CRC and OC. The SWI/SNF chromatin remodeling gene ARID1A was significantly associated with TMB in CRC, OC, and PC, and SMARCA4 mutations were significantly linked to TMB in CRC. The significant associations of ATR mutations, a DNA damage sensor gene, with TMB were observed in CRC and PC. In CRC, significant associations of HR (BRCA1, BRCA2, BARD1, PALB2, and BRIP1) and Fanconi anemia (FANCA, FANCC, and FANCG) genes with TMB were identified. This suggests a CRC-specific connection between these repair processes and TMB. Although many cancers harbored DDR GAs, the frequency of GAs did not always correlate with median TMB For example, CRC, OC, and PC were enriched with multiple DDR GAs, which might lead to their high TMB, while BC and NSCLC showed high TMB but fewer enriched DDR GAs. These variances suggest that the connection between DDR GAs and TMB is multifaceted.
- Tumors with ATM GAs were more prevalent in high TMB tumor types, suggesting a potential avenue for synthetic lethality strategies with ATR inhibitors. ARID1A GAs, frequent in BC, NSCLC, CRC, and OC, correlated with TMB-high tumors, reinforcing the potential of combining immunotherapy with ATR inhibitors in these scenarios.
- Associations between DDR gene mutations and elevated levels of PD-L1 expression (DAKO 22C3 core cut-off = TPS 1 for CRC, OC, and PC; CPS 10 for BC; and TPS 50 for NSCLC) turned out to be not only rare but also specific to particular tumor types. TP53 mutations associated with PD-L1-high status in BC, NSCLC, and OC, but not in CRC or PC. The only other potent associations between genes and PD-L1-high status observed in these five tumor types were for ARID1A and MSH6 in CRC and MSH2 in PC No significant correlations between genes grouped according to DDR pathway and PD-L1-high status were observed.
- The relationship between high PD-L1 expression and high TMB was weak, reemphasizing that they serve as independent predictive biomarkers for the utilization of ICIs.
Take-Home Message and Future Directions
- This study emphasizes the comprehensive relationship among DDR GAs, TMB, and PD-L1 status in various cancers. Although most tumors harbor DDR GAs, there is significant variation in the genomic landscape across different tumor types. It is crucial to identify associated DDR genes as predictive biomarkers, enabling indication-specific, real-world clinical genomic data-based precision oncology.
- This approach could guide the selective combination of DDR inhibitors with immune checkpoint inhibitors (ICIs) to enhance clinical outcomes. The findings offer solutions to intriguing clinical hypotheses. For instance, the association between mutations in SWI/SNF chromatin remodeling genes, ARID1A and SMARCA4, as well as ATR, with high TMB presents an opportunity for further clinical investigation. In certain tumors, like NSCLC, these mutations may serve as additional biomarkers for ICI therapy. Combining ATR inhibitors with ICIs in tumors with these GAs could be a promising strategy.
- In conclusion, the insights gained from these results, derived from clinically available and actionable assays, further the understanding of the interplay between DDR GAs in cancer. This enhanced understanding not only promotes the exploration of immune checkpoint inhibitors in specific tumor types and biomarker-defined subgroups (e.g., those with mutations in ARID1A, SMARCA4, or ATR) but also supports the combination of ICIs with ATR inhibitors in a clinical environment.
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
AI Approaches in Drug Design
Publishing Model: Open Access
Deadline: Mar 31, 2026
Genomic Instability
Publishing Model: Open Access
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in