Resolving the prenatal events of KMT2A::AFF1-driven disease initiation
Published in Cancer, Biomedical Research, and General & Internal Medicine
Despite a remarkable progress in treating children with leukemia, infants with acute lymphoblastic leukemia (ALL) driven by the KMT2A::AFF1-fusion still have a poor prognosis. We now provide key insights into the KMT2A::AFF1 pre-leukemic phase using a novel murine model, of critical importance to human disease initiation.
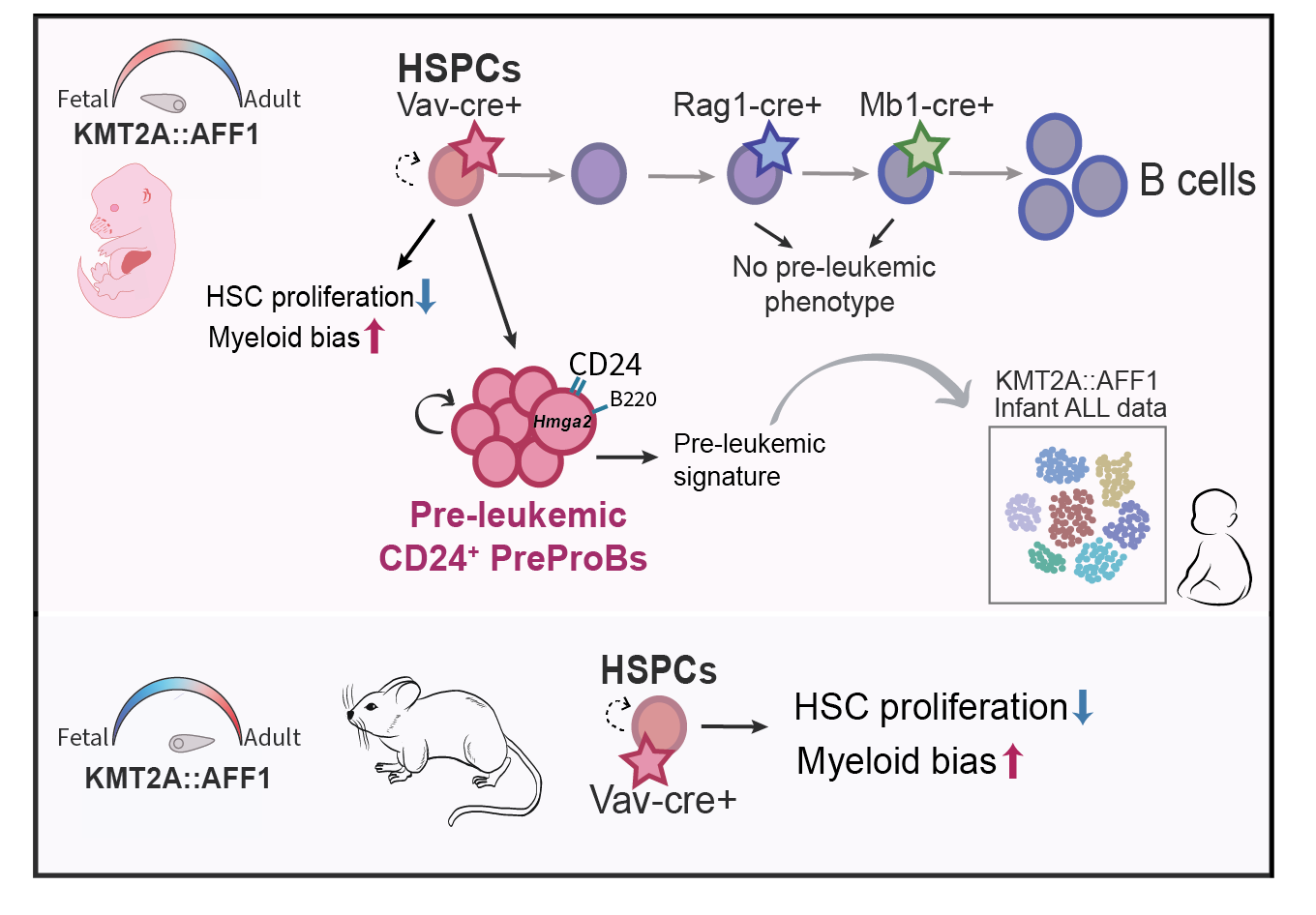
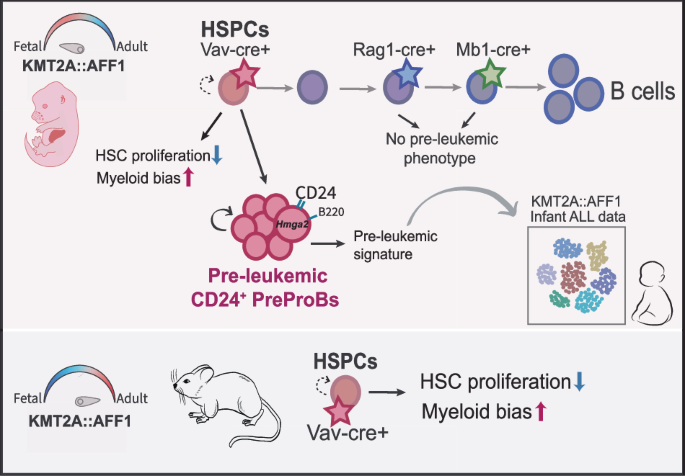
The KMT2A::AFF1 translocation occurs in an embryonic context and to uncover the prenatal events driving disease development, we have generated a Cre-inducible KMT2A::AFF1 model, enabling precise targeting of the fusion gene to embryonic hematopoietic progenitors. This approach revealed a striking pre-leukemic state, that was confined to embryonic induction in hematopoietic stem and progenitor cells (HSPCS). These pre-leukemic cells were immunophenotypically PreProB cells (B220+CD19-), but notably marked by CD24 surface expression. The subset had lineage plasticity, aberrant long-term engraftment capacity and pathogenic characteristics consistent with pre-leukemic stem cells. Molecular analysis revealed a pre-malignant profile and expression of stemness genes, such as Hmga2, which is also associated with fetal hematopoiesis. Importantly, a subset of cells expressing CD24 and HMGA2, could be observed in KMT2A::AFF1 patients, linking our model to human disease.
The pre-leukemic state could not be seen upon post-natal induction. However, there was an effect on the HSPC compartment with a bias towards myeloid output in vitro and a selective negative impact on the cloning capacity of hematopoietic stem cells (HSCs). This effect was independent of developmental state. Additionally, induction of the oncogene in more lymphoid committed progenitors did not give rise to any pre-leukemic phenotype, highlighting the importance of cell of origin and developmental state for disease induction.
Overall, our novel model reveals a unique pre-leukemic state, of critical importance to human disease initiation.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in