Rooted Revelations: Unearthing the Mechanistic Secrets of Ibogaine
Published in Biomedical Research and Pharmacy & Pharmacology

The roots of the Tabernanthe iboga plant, native to Gabon, are rich in ibogaine, a natural alkaloid known for its hallucinogenic effects and promising properties in treating substance use disorders. This potential application was serendipitously discovered in 1962 by Howard Lotsof, who, along with other heroin users, initially experimented with ibogaine for its psychoactive properties. Remarkably, they reported an absence of opioid withdrawal symptoms for several days following the exposure.
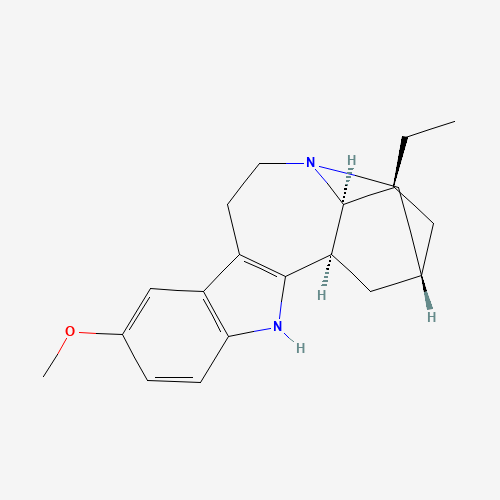 Figure 1. Ibogaine molecule.
Figure 1. Ibogaine molecule.
Ibogaine's history dates back even further than commonly thought, with its isolation from T. iboga occurring in 1900. Early on, it was discovered that ibogaine had a topical anesthetic effect similar to cocaine, and stimulant effects akin to those of the kola nut. Additionally, it was reported to have aphrodisiac effects. Given the popularity of coca and kola at the time, along with its effects on libido, one might assume that ibogaine would have been widely sold. However, factors such as the stigma surrounding African drugs, supply difficulties, and a general lack of medical interest limited its commercialization. Despite these challenges, various products claiming benefits for neurasthenia, depression, fatigue, or viral infections did appear on the French market.
Over the past few decades, research on this molecule has been primarily limited due to its questionable safety profile — it is a QT-prolonging drug, and cases of fatalities associated with its use are frequently reported in the literature- as well as its complex mechanism of action. However, the urgent need to tackle substance use disorders, particularly opioid use disorder in contexts like the United States and Canada, has renewed interest in the therapeutic potential of this molecule.
In Catalonia, Spain, in the context of the studies belonging to a doctoral thesis, we are providing valuable data on the mechanisms of action of ibogaine, first collecting and updating everything we know about its binding profile, but also conducting analyses through transcriptomic approaches. Regarding its binding profile, we have demonstrated how ibogaine can modulate multiple receptor systems, most of which are involved in the development and establishment of substance use disorders. Thus, ibogaine appears to be highly valuable due to its ability to correct the dysfunctional activity of several targets and steer them away from patterns of drug dependence. We can see a clinical correlate of this broad effect when ibogaine is able to reduce cravings, but also tolerance and the withdrawal syndrome of different drugs.
In our transcriptomic approach, we analyzed the brain cortical tissue of mice acutely exposed to ibogaine using transcriptomic analysis. This technique enables the identification of significant gene encoding changes associated with various conditions, including acute drug exposure. Notably, this is the first application of this technique in studying ibogaine's mechanisms of action. The results suggest involvement of hormones like vasopressin or oxytocin, or processes such as synapse formation, in ibogaine's therapeutic effects. A particularly interesting finding is the gender-specific response: while males showed significant changes in eight genes, females exhibited significant changes in 28 genes. This aligns with previous studies that report enhanced bioavailability of ibogaine in females, which could have significant implications for human studies. These preliminary findings regarding new mechanisms and gender differences warrant further investigation in future research.
In addition to these studies, our project includes the first Phase-II clinical trial on ibogaine, which, at the time of writing this, is nearing completion. The trial recruited 20 participants enrolled in methadone maintenance programs who wished to discontinue their methadone use. We specifically chose this profile because many individuals in these programs find it extremely difficult to cease methadone use due to opioid withdrawal syndrome, which often results in prolonged iatrogenic drug dependence.
All these studies except the preclinical research are being designed and conducted from the NGO ICEERS in collaboration with the Universitat Rovira i Virgili, the Autonomous University of Madrid (Spain) and the University of São Paulo (Brazil), without the involvement of pharmaceutical industry. Extreme caution was exercised at all stages of our research, considering the nature of the product. Ibogaine is derived from the genetic resources of Gabon and is found in Tabernanthe iboga, a plant integral to the rich and ancestral ceremonies of certain local traditions. The growing international interest in ibogaine has threatened T. iboga populations. Therefore, we used ibogaine extracted from Voacanga africana, a non-endangered plant, as an alternative. It is imperative to consider the ethical and sustainability impacts when researching natural products with traditional uses. There are numerous examples of how improper scientific practices have led to the impoverishment, restricted access, or even complete eradication of natural products, adversely affecting local contexts.
.jpeg)






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in